Requirement for neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum
- PMID: 12960419
- PMCID: PMC284799
- DOI: 10.1091/mbc.e03-07-0463
Requirement for neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum
Abstract
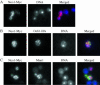
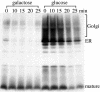
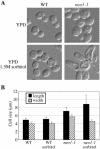
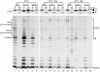
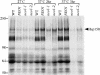
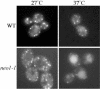
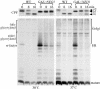
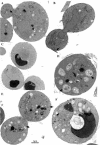
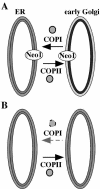
Neo1p from Saccharomyces cerevisiae is an essential P-type ATPase and potential aminophospholipid translocase (flippase) in the Drs2p family. We have previously implicated Drs2p in protein transport steps in the late secretory pathway requiring ADP-ribosylation factor (ARF) and clathrin. Here, we present evidence that epitope-tagged Neo1p localizes to the endoplasmic reticulum (ER) and Golgi complex and is required for a retrograde transport pathway between these organelles. Using conditional alleles of NEO1, we find that loss of Neo1p function causes cargo-specific defects in anterograde protein transport early in the secretory pathway and perturbs glycosylation in the Golgi complex. Rer1-GFP, a protein that cycles between the ER and Golgi complex in COPI and COPII vesicles, is mislocalized to the vacuole in neo1-ts at the nonpermissive temperature. These phenotypes suggest that the anterograde protein transport defect is a secondary consequence of a defect in a COPI-dependent retrograde pathway. We propose that loss of lipid asymmetry in the cis Golgi perturbs retrograde protein transport to the ER.
Figures



References
-
- Balasubramanian, K., and Schroit, A.J. (2003). Aminophospholipid asymmetry: a matter of life and death. Annu. Rev. Physiol. 65, 701-734. - PubMed
-
- Barlowe, C. (2002). COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol. 14, 417-422. - PubMed
-
- Barlowe, C., and Schekman, R. (1993). SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365, 347-349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

