The simultaneous production of phosphatidic acid and diacylglycerol is essential for the translocation of protein kinase Cepsilon to the plasma membrane in RBL-2H3 cells
- PMID: 12960426
- PMCID: PMC284792
- DOI: 10.1091/mbc.e03-05-0295
The simultaneous production of phosphatidic acid and diacylglycerol is essential for the translocation of protein kinase Cepsilon to the plasma membrane in RBL-2H3 cells
Abstract
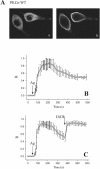
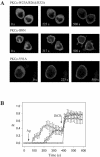
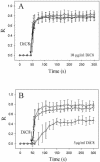
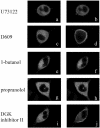
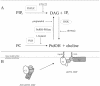
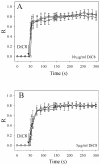
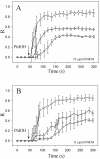
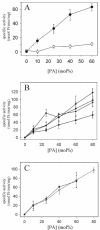
To evaluate the role of the C2 domain in protein kinase Cepsilon (PKCepsilon) localization and activation after stimulation of the IgE receptor in RBL-2H3 cells, we used a series of mutants located in the phospholipid binding region of the enzyme. The results obtained suggest that the interaction of the C2 domain with the phospholipids in the plasma membrane is essential for anchoring the enzyme in this cellular compartment. Furthermore, the use of specific inhibitors of the different pathways that generate both diacylglycerol and phosphatidic acid has shown that the phosphatidic acid generated via phospholipase D (PLD)-dependent pathway, in addition to the diacylglycerol generated via phosphoinosite-phospholipase C (PLC), are involved in the localization of PKCepsilon in the plasma membrane. Direct stimulation of RBL-2H3 cells with very low concentrations of permeable phosphatidic acid and diacylglycerol exerted a synergistic effect on the plasma membrane localization of PKCepsilon. Moreover, the in vitro kinase assays showed that both phosphatidic acid and diacylglycerol are essential for enzyme activation. Together, these results demonstrate that phosphatidic acid is an important and essential activator of PKCepsilon through the C2 domain and locate this isoenzyme in a new scenario where it acts as a downstream target of PLD.
Figures


References
-
- Akita, Y., et al. (1994). Overproduction of a Ca2+-independent protein kinase C isozyme, nPKC epsilon, increases the secretion of prolactin from thyrotropin-releasing hormone-stimulated rat pituitary GH4C1 cells. J. Biol. Chem. 269, 4653–4660. - PubMed
-
- Andresen, B.T., Rizzo, M.A., Shome, K., and Romero, G. (2002). The role of phosphatidic acid in the regulaton of the Ras/MEK/ERK signalling cascade. FEBS Lett. 531, 65–68. - PubMed
-
- Besson, A., Wilson, T.L., and Yong, V.W. (2002). The anchoring protein RACK1 links protein kinase C epsilon to integrin beta chains. J. Biol. Chem. 277, 22073–22084. - PubMed
-
- Bleasdale, J.E., Thakur, N.R., Gremban, R.S., Bundy, G.L., Fitzpatrick, F.A., Smith, R.J., and Bunting, S. (1990). Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 255, 756–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

