Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction
- PMID: 12960430
- PMCID: PMC284780
- DOI: 10.1091/mbc.e03-04-0205
Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction
Abstract
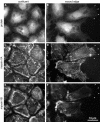
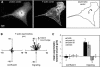
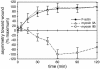
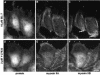
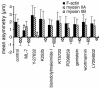
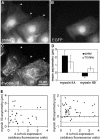
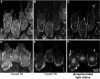
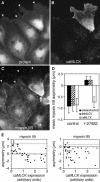
All vertebrates contain two nonmuscle myosin II heavy chains, A and B, which differ in tissue expression and subcellular distributions. To understand how these distinct distributions are controlled and what role they play in cell migration, myosin IIA and IIB were examined during wound healing by bovine aortic endothelial cells. Immunofluorescence showed that myosin IIA skewed toward the front of migrating cells, coincident with actin assembly at the leading edge, whereas myosin IIB accumulated in the rear 15-30 min later. Inhibition of myosin light-chain kinase, protein kinases A, C, and G, tyrosine kinase, MAP kinase, and PIP3 kinase did not affect this asymmetric redistribution of myosin isoforms. However, posterior accumulation of myosin IIB, but not anterior distribution of myosin IIA, was inhibited by dominant-negative rhoA and by the rho-kinase inhibitor, Y-27632, which also inhibited myosin light-chain phosphorylation. This inhibition was overcome by transfecting cells with constitutively active myosin light-chain kinase. These observations indicate that asymmetry of myosin IIB, but not IIA, is regulated by light-chain phosphorylation mediated by rho-dependent kinase. Blocking this pathway inhibited tail constriction and retraction, but did not affect protrusion, suggesting that myosin IIB functions in pulling the rear of the cell forward.
Figures


Similar articles
-
Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration.Cancer Res. 2006 May 1;66(9):4725-33. doi: 10.1158/0008-5472.CAN-05-4236. Cancer Res. 2006. PMID: 16651425
-
Myosin IIA drives neurite retraction.Mol Biol Cell. 2003 Nov;14(11):4654-66. doi: 10.1091/mbc.e03-03-0187. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960431 Free PMC article.
-
The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells.Mol Biol Cell. 2008 Dec;19(12):5156-67. doi: 10.1091/mbc.e08-05-0533. Epub 2008 Oct 8. Mol Biol Cell. 2008. PMID: 18843042 Free PMC article.
-
Nonmuscle myosin-2: mix and match.Cell Mol Life Sci. 2013 Jan;70(1):1-21. doi: 10.1007/s00018-012-1002-9. Epub 2012 May 8. Cell Mol Life Sci. 2013. PMID: 22565821 Free PMC article. Review.
-
Mammalian nonmuscle myosin II comes in three flavors.Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
Cited by
-
YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility.J Cell Biol. 2019 Apr 1;218(4):1369-1389. doi: 10.1083/jcb.201806065. Epub 2019 Feb 8. J Cell Biol. 2019. PMID: 30737263 Free PMC article.
-
Nonmuscle myosin is regulated during smooth muscle contraction.Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H191-9. doi: 10.1152/ajpheart.00132.2009. Epub 2009 May 8. Am J Physiol Heart Circ Physiol. 2009. PMID: 19429828 Free PMC article.
-
Kif26b controls endothelial cell polarity through the Dishevelled/Daam1-dependent planar cell polarity-signaling pathway.Mol Biol Cell. 2016 Mar 15;27(6):941-53. doi: 10.1091/mbc.E14-08-1332. Epub 2016 Jan 20. Mol Biol Cell. 2016. PMID: 26792835 Free PMC article.
-
Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders.Pharmacol Rev. 2016 Apr;68(2):476-532. doi: 10.1124/pr.115.010652. Pharmacol Rev. 2016. PMID: 27037223 Free PMC article. Review.
-
Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility.Am J Pathol. 2007 Nov;171(5):1640-9. doi: 10.2353/ajpath.2007.070325. Epub 2007 Sep 14. Am J Pathol. 2007. PMID: 17872978 Free PMC article.
References
-
- Amano, M., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., and Kaibuchi, K. (1997). Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science 275, 1308–1311. - PubMed
-
- Amano, M., Itoh, M., Kimura, K., Fukata, Y., Chihara, K., Nakano, T., Matsuura, Y., and Kaibuchi, K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246–20249. - PubMed
-
- Bhatia-Dey, N., Taira, M., Conti, M.A., Nooruddin, H., and Adelstein, R.S. (1998). Differential expression of non-muscle myosin heavy chain genes during Xenopus embryogenesis. Mech. Dev. 78, 33–36. - PubMed
-
- Choi, O.H., Park, C.S., Itoh, K., Adelstein, R.S., and Beaven, M.A. (1996). Cloning of the cDNA encoding rat myosin heavy chain-A and evidence for the absence of myosin heavy chain-B in cultured rat mast (RBL-2H3) cells. J Muscle Res. Cell Motil. 17, 69–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

