Myosin IIA drives neurite retraction
- PMID: 12960431
- PMCID: PMC266780
- DOI: 10.1091/mbc.e03-03-0187
Myosin IIA drives neurite retraction
Abstract
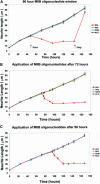
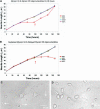
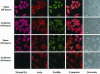
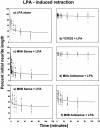
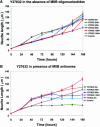
Neuritic extension is the resultant of two vectorial processes: outgrowth and retraction. Whereas myosin IIB is required for neurite outgrowth, retraction is driven by a motor whose identity has remained unknown until now. Preformed neurites in mouse Neuro-2A neuroblastoma cells undergo immediate retraction when exposed to isoform-specific antisense oligonucleotides that suppress myosin IIB expression, ruling out myosin IIB as the retraction motor. When cells were preincubated with antisense oligonucleotides targeting myosin IIA, simultaneous or subsequent addition of myosin IIB antisense oligonucleotides did not elicit neurite retraction, both outgrowth and retraction being curtailed. Even during simultaneous application of antisense oligonucleotides against both myosin isoforms, lamellipodial spreading continued despite the complete inhibition of neurite extension, indicating an uncoupling of lamellipodial dynamics from movement of the neurite. Significantly, lysophosphatidate- or thrombin-induced neurite retraction was blocked not only by the Rho-kinase inhibitor Y27632 but also by antisense oligonucleotides targeting myosin IIA. Control oligonucleotides or antisense oligonucleotides targeting myosin IIB had no effect. In contrast, Y27632 did not inhibit outgrowth, a myosin IIB-dependent process. We conclude that the conventional myosin motor, myosin IIA, drives neurite retraction.
Figures


References
-
- Amano, M., Chihara, K., Nakamura, N., Fukata, Y., Yano, T., Shibata, M., Ikebe, M., and Kaibuchi, K. (1998). Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 3, 177-188. - PubMed
-
- Berg, J.S., and Cheney, R.E. (2002). Myosin X is an unconventional myosin that undergoes intrafilopodial motility. Nat. Cell Biol. 4, 246-250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

