Intersectin regulates fission and internalization of caveolae in endothelial cells
- PMID: 12960435
- PMCID: PMC284801
- DOI: 10.1091/mbc.e03-01-0041
Intersectin regulates fission and internalization of caveolae in endothelial cells
Abstract
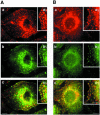
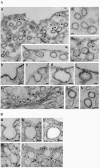
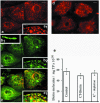
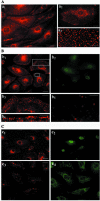
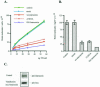
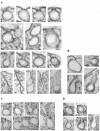
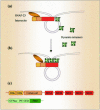
Intersectin, a multiple Eps15 homology and Src homology 3 (SH3) domain-containing protein, is a component of the endocytic machinery in neurons and nonneuronal cells. However, its role in endocytosis via caveolae in endothelial cells (ECs) is unclear. We demonstrate herein by coimmunoprecipitation, velocity sedimentation on glycerol gradients, and cross-linking that intersectin is present in ECs in a membrane-associated protein complex containing dynamin and SNAP-23. Electron microscopy (EM) immunogold labeling studies indicated that intersectin associated preferentially with the caveolar necks, and it remained associated with caveolae after their fission from the plasmalemma. A cell-free system depleted of intersectin failed to support caveolae fission from the plasma membrane. A biotin assay used to quantify caveolae internalization and extensive EM morphological analysis of ECs overexpressing wt-intersectin indicated a wide range of morphological changes (i.e., large caveolae clusters marginated at cell periphery and pleiomorphic caveolar necks) as well as impaired caveolae internalization. Biochemical evaluation of caveolae-mediated uptake by ELISA showed a 68.4% inhibition by reference to control. We also showed that intersectin interaction with dynamin was important in regulating the fission and internalization of caveolae. Taken together, the results indicate the crucial role of intersectin in the mechanism of caveolae fission in endothelial cells.
Figures
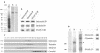


References
-
- Adams, A., Thorn, J.M., Yamabhai, M., Kay, B.K., and O'Brian, J.P. (2000). Intersectin, an adaptor protein involved in clathrin-mediated endocytosis activates mitogenic signaling pathways. J. Biol. Chem. 275, 27414-27420. - PubMed
-
- Anderson, E., Hellman, L., Gullberg, U., and Olsson, I. (1998). The role of the propeptide for processing and sorting of human myeloperoxidase. J. Biol. Chem. 273, 4747-4753. - PubMed
-
- Boyles, J., L'Hernault, N., Laks, H., and Palade, G.E. (1981). Evidence for a vesicular shuttle in heart capillaries. J. Cell Biol. 91, 418a.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

