The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa
- PMID: 12960438
- PMCID: PMC266756
- DOI: 10.1091/mbc.e02-07-0433
The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa
Abstract
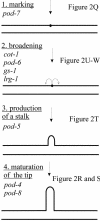
Cellular polarity is a fundamental property of every cell. Due to their extremely fast growth rate (>/=1 microm/s) and their highly elongated form, filamentous fungi represent a prime example of polarized growth and are an attractive model for the analysis of fundamental mechanisms underlying cellular polarity. To identify the critical components that contribute to polarized growth, we developed a large-scale genetic screen for the isolation of conditional mutants defective in this process in the model fungus Neurospora crassa. Phenotypic analysis and complementation tests of ca. 950 mutants identified more than 100 complementation groups that define 21 distinct morphological classes. The phenotypes include polarity defects over the whole hypha, more specific defects localized to hyphal tips or subapical regions, and defects in branch formation and growth directionality. To begin converting this mutant collection into meaningful biological information, we identified the defective genes in 45 mutants covering all phenotypic classes. These genes encode novel proteins as well as proteins which 1) regulate the actin or microtubule cytoskeleton, 2) are kinases or components of signal transduction pathways, 3) are part of the secretory pathway, or 4) have functions in cell wall formation or membrane biosynthesis. These findings highlight the dynamic nature of a fungal hypha and establish a molecular model for studies of hyphal growth and polarity.
Figures
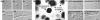

Similar articles
-
Architecture and development of the Neurospora crassa hypha -- a model cell for polarized growth.Fungal Biol. 2011 Jun;115(6):446-74. doi: 10.1016/j.funbio.2011.02.008. Epub 2011 Feb 19. Fungal Biol. 2011. PMID: 21640311 Review.
-
A mutation in the Neurospora crassa actin gene results in multiple defects in tip growth and branching.Fungal Genet Biol. 2004 Feb;41(2):213-25. doi: 10.1016/j.fgb.2003.10.010. Fungal Genet Biol. 2004. PMID: 14732267
-
Cell fusion in the filamentous fungus, Neurospora crassa.Methods Mol Biol. 2008;475:21-38. doi: 10.1007/978-1-59745-250-2_2. Methods Mol Biol. 2008. PMID: 18979236 Review.
-
Coronin is a component of the endocytic collar of hyphae of Neurospora crassa and is necessary for normal growth and morphogenesis.PLoS One. 2012;7(5):e38237. doi: 10.1371/journal.pone.0038237. Epub 2012 May 31. PLoS One. 2012. PMID: 22693603 Free PMC article.
-
The actin motor MYO-5 effect in the intracellular organization of Neurospora crassa.Fungal Genet Biol. 2019 Apr;125:13-27. doi: 10.1016/j.fgb.2018.11.008. Epub 2019 Jan 4. Fungal Genet Biol. 2019. PMID: 30615944
Cited by
-
Reversibility of motor dysfunction in the rat model of NGLY1 deficiency.Mol Brain. 2021 Jun 13;14(1):91. doi: 10.1186/s13041-021-00806-6. Mol Brain. 2021. PMID: 34120625 Free PMC article.
-
The essential phosphoinositide kinase MSS-4 is required for polar hyphal morphogenesis, localizing to sites of growth and cell fusion in Neurospora crassa.PLoS One. 2012;7(12):e51454. doi: 10.1371/journal.pone.0051454. Epub 2012 Dec 13. PLoS One. 2012. PMID: 23272106 Free PMC article.
-
Synergistic Inhibition of Mycotoxigenic Fungi and Mycotoxin Production by Combination of Pomegranate Peel Extract and Azole Fungicide.Front Microbiol. 2019 Aug 20;10:1919. doi: 10.3389/fmicb.2019.01919. eCollection 2019. Front Microbiol. 2019. PMID: 31481948 Free PMC article.
-
Analysis of mutations in Neurospora crassa ERMES components reveals specific functions related to β-barrel protein assembly and maintenance of mitochondrial morphology.PLoS One. 2013 Aug 5;8(8):e71837. doi: 10.1371/journal.pone.0071837. Print 2013. PLoS One. 2013. PMID: 23940790 Free PMC article.
-
Establishment of Neurospora crassa as a host for heterologous protein production using a human antibody fragment as a model product.Microb Cell Fact. 2017 Jul 25;16(1):128. doi: 10.1186/s12934-017-0734-5. Microb Cell Fact. 2017. PMID: 28743272 Free PMC article.
References
-
- Adams, M.D. et al. (2000). The genome sequence of Drosophila melanogaster. Science 287, 2185-2195. - PubMed
-
- Bartnicki-Garcia, S. (1973). Fundamental aspects of hyphal morphogenesis. In: Microbial Differentiation, ed. J.M. Ashworth and J.E. Smith, Cambridge University Press, 245-267.
-
- Bartnicki-Garcia, S. (1999). Glucans, walls, and morphogenesis: on the contributions of J. G. H. Wessels to the golden decades of fungal physiology and beyond. Fungal Genet. Biol. 27, 119-127. - PubMed
-
- Bartnicki-Garcia, S., Hergert, F., and Gierz, G. (1989). Computer simulation of fungal morphogenesis and the mathematical basis for hyphal (tip) growth. Protoplasma 153, 46-57.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

