A perfect correlate does not a surrogate make
- PMID: 12962545
- PMCID: PMC212489
- DOI: 10.1186/1471-2288-3-16
A perfect correlate does not a surrogate make
Abstract
Background: There is common belief among some medical researchers that if a potential surrogate endpoint is highly correlated with a true endpoint, then a positive (or negative) difference in potential surrogate endpoints between randomization groups would imply a positive (or negative) difference in unobserved true endpoints between randomization groups. We investigate this belief when the potential surrogate and unobserved true endpoints are perfectly correlated within each randomization group.
Methods: We use a graphical approach. The vertical axis is the unobserved true endpoint and the horizontal axis is the potential surrogate endpoint. Perfect correlation within each randomization group implies that, for each randomization group, potential surrogate and true endpoints are related by a straight line. In this scenario the investigator does not know the slopes or intercepts. We consider a plausible example where the slope of the line is higher for the experimental group than for the control group.
Results: In our example with unknown lines, a decrease in mean potential surrogate endpoints from control to experimental groups corresponds to an increase in mean true endpoint from control to experimental groups. Thus the potential surrogate endpoints give the wrong inference. Similar results hold for binary potential surrogate and true outcomes (although the notion of correlation does not apply). The potential surrogate endpoint would give the correct inference if either (i) the unknown lines for the two group coincided, which means that the distribution of true endpoint conditional on potential surrogate endpoint does not depend on treatment group, which is called the Prentice Criterion or (ii) if one could accurately predict the lines based on data from prior studies.
Conclusion: Perfect correlation between potential surrogate and unobserved true outcomes within randomized groups does not guarantee correct inference based on a potential surrogate endpoint. Even in early phase trials, investigators should not base conclusions on potential surrogate endpoints in which the only validation is high correlation with the true endpoint within a group.
Figures
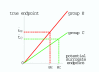
 is smaller than the mean surrogate outcome in the C group
is smaller than the mean surrogate outcome in the C group  . However the mean true outcome in the E group
. However the mean true outcome in the E group  is larger than the mean true outcome in the C group,
is larger than the mean true outcome in the C group,  , yielding the opposite conclusion for the effect of experimental intervention.
, yielding the opposite conclusion for the effect of experimental intervention.References
-
- Fleming TR, DeMets DL. Surrogate end points in clinical trials: Are we being misled? Annals of Internal Medicine. 1996;125:605–613. - PubMed
-
- Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative Randomized Controlled Trial. Journal of the American Medical Association. 2002;288:2321–333. doi: 10.1001/jama.288.3.321. - DOI - PubMed
-
- Sandier RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New England Journal of Medicine. 2003;348:883–890. doi: 10.1056/NEJMoa021633. - DOI - PubMed
-
- Baron JA, Cole BF, Sandier RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU. A randomized trial of aspirin to prevent colorectal adenomas. New England Journal of Medicine. 2003;348:891–899. doi: 10.1056/NEJMoa021735. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

