Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II
- PMID: 12962548
- PMCID: PMC200965
- DOI: 10.1186/1471-2091-4-10
Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II
Abstract
Background: Annexin II heavy chain (also called p36, calpactin I) is lost in prostate cancers and in a majority of prostate intraepithelial neoplasia (PIN). Loss of annexin II heavy chain appears to be specific for prostate cancer since overexpression of annexin II is observed in a majority of human cancers, including pancreatic cancer, breast cancer and brain tumors. Annexin II exists as a heterotetramer in complex with a protein ligand p11 (S100A10), and as a monomer. Diverse cellular functions are proposed for the two forms of annexin II. The monomer is involved in DNA synthesis. A leucine-rich nuclear export signal (NES) in the N-terminus of annexin II regulates its nuclear export by the CRM1-mediated nuclear export pathway. Mutation of the NES sequence results in nuclear retention of annexin II.
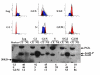
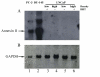
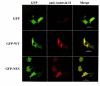
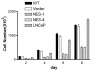
Results: Annexin II localized in the nucleus is phosphorylated, and the appearance of nuclear phosphorylated annexin II is cell cycle dependent, indicating that phosphorylation may play a role in nuclear entry, retention or export of annexin II. By exogenous expression of annexin II in the annexin II-null LNCaP cells, we show that wild-type annexin II is excluded from the nucleus, whereas the NES mutant annexin II localizes in both the nucleus and cytoplasm. Nuclear retention of annexin II results in reduced cell proliferation and increased doubling time of cells. Expression of annexin II, both wild type and NES mutant, causes morphological changes of the cells. By site-specific substitution of glutamic acid in the place of serines 11 and 25 in the N-terminus, we show that simultaneous phosphorylation of both serines 11 and 25, but not either one alone, prevents nuclear localization of annexin II.
Conclusion: Our data show that nuclear annexin II is phosphorylated in a cell cycle-dependent manner and that substitution of serines 11 and 25 inhibit nuclear entry of annexin II. Aberrant accumulation of nuclear annexin II retards proliferation of LNCaP cells.
Figures




Similar articles
-
Regulation of nucleo-cytoplasmic shuttling of human annexin A2: a proposed mechanism.Mol Cell Biochem. 2007 Sep;303(1-2):211-20. doi: 10.1007/s11010-007-9477-7. Epub 2007 Apr 25. Mol Cell Biochem. 2007. PMID: 17457518
-
Functional interaction of the Ras effector RASSF5 with the tyrosine kinase Lck: critical role in nucleocytoplasmic transport and cell cycle regulation.J Mol Biol. 2010 Mar 19;397(1):89-109. doi: 10.1016/j.jmb.2010.01.005. Epub 2010 Jan 11. J Mol Biol. 2010. PMID: 20064523
-
Extracellular signal-regulated kinase 2 (ERK-2) mediated phosphorylation regulates nucleo-cytoplasmic shuttling and cell growth control of Ras-associated tumor suppressor protein, RASSF2.Exp Cell Res. 2009 Oct 1;315(16):2775-90. doi: 10.1016/j.yexcr.2009.06.013. Epub 2009 Jun 23. Exp Cell Res. 2009. PMID: 19555684
-
AMPK Localization: A Key to Differential Energy Regulation.Int J Mol Sci. 2021 Oct 10;22(20):10921. doi: 10.3390/ijms222010921. Int J Mol Sci. 2021. PMID: 34681581 Free PMC article. Review.
-
Molecular pathways involved in the transport of nuclear receptors from the nucleus to cytoplasm.J Steroid Biochem Mol Biol. 2018 Apr;178:36-44. doi: 10.1016/j.jsbmb.2017.10.020. Epub 2017 Oct 26. J Steroid Biochem Mol Biol. 2018. PMID: 29107180 Review.
Cited by
-
Annexin A2: its molecular regulation and cellular expression in cancer development.Dis Markers. 2014;2014:308976. doi: 10.1155/2014/308976. Epub 2014 Jan 23. Dis Markers. 2014. PMID: 24591759 Free PMC article. Review.
-
Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes.J Virol. 2013 Jul;87(13):7502-15. doi: 10.1128/JVI.00519-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637395 Free PMC article.
-
Expression of biomarkers modulating prostate cancer angiogenesis: differential expression of annexin II in prostate carcinomas from India and USA.Mol Cancer. 2003 Oct 8;2:34. doi: 10.1186/1476-4598-2-34. Mol Cancer. 2003. PMID: 14613585 Free PMC article.
-
Efficient nanoparticle mediated sustained RNA interference in human primary endothelial cells.Nanotechnology. 2011 Nov 4;22(44):445101. doi: 10.1088/0957-4484/22/44/445101. Epub 2011 Oct 11. Nanotechnology. 2011. PMID: 21990205 Free PMC article.
-
A competitive hexapeptide inhibitor of annexin A2 prevents hypoxia-induced angiogenic events.J Cell Sci. 2011 May 1;124(Pt 9):1453-64. doi: 10.1242/jcs.079236. Epub 2011 Apr 12. J Cell Sci. 2011. PMID: 21486955 Free PMC article.
References
-
- Geisow MJ, Walker JH, Boustead C, Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987;7:289–298. - PubMed
-
- Thiel C, Osborn M, Gerke V. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11- and Ca(2+)-binding sites. J Cell Sci. 1992;103 ( Pt 3):733–742. - PubMed
-
- Creutz CE. The annexins and exocytosis. Science. 1992;258:924–931. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

