The effects of spermine on the accessibility of residues in the M2 segment of Kir2.1 channels expressed in Xenopus oocytes
- PMID: 12963788
- PMCID: PMC2343490
- DOI: 10.1113/jphysiol.2003.052845
The effects of spermine on the accessibility of residues in the M2 segment of Kir2.1 channels expressed in Xenopus oocytes
Abstract
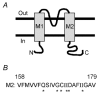
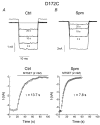
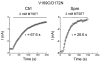
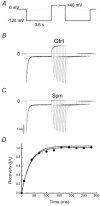
We examined the effects of spermine binding to aspartate at site 172 on the accessibility of internal trimethylammonioethylmethane thiosulphonate (MTSET) to substituted cysteines within the pore of a Kir2.1 channel. Spermine prevented MTSET modification in Q164C and G168C mutants, indicating that sites 164 and 168 are located externally to the spermine binding site. The rates of MTSET modification were significantly reduced by spermine in I176C mutants, indicating that site 176 is located internally to D172 and that the bound spermine hinders the reaction of MTSET with cysteine at site 176. Spermidine, putrescine and Mg2+ also decreased MTSET modification at site 176. The order of effect is putrescine > spermidine approximately = spermine approximately = Mg2+. To account for the electrostatic and physical repulsion between MTSET and polyamines, possible locations of polyamines in the pore are discussed. In D172C mutants, the spermine that bound to sites 224 and 299 completely inhibited channels at +40 mV, yet MTSET remained accessible to site 172. In addition, in the D172C mutant, spermine did not affect the exit rate of Ba2+ bound to the threonine at the site 141. These results indicate that spermine bound at the cytoplasmic pore induces channel closure at positions 141-172. The effects of spermine on the accessibility of amino acids in the pore may shed light on the structural and functional relationships of the Kir2.1 channels during inward rectification.
Figures





References
-
- Hamill OP, Marty A, Heher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

