Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex
- PMID: 12963791
- PMCID: PMC2343567
- DOI: 10.1113/jphysiol.2003.050153
Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex
Abstract
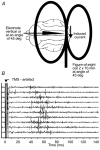
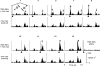
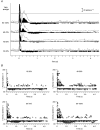
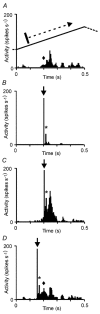
Transcranial magnetic stimulation (TMS) has become a well established procedure for testing and modulating the neuronal excitability of human brain areas, but relatively little is known about the cellular processes induced by this rather coarse stimulus. In a first attempt, we performed extracellular single-unit recordings in the primary visual cortex (area 17) of the anaesthetised and paralysed cat, with the stimulating magnetic field centred at the recording site (2 x 70 mm figure-of-eight coil). The effect of single biphasic TMS pulses, which induce a lateral-to-medial electric current within the occipital pole of the right hemisphere, was tested for spontaneous as well as visually evoked activity. For cat visual cortex we found that a single TMS pulse elicited distinct episodes of enhanced and suppressed activity: in general, a facilitation of activity was found during the first 500 ms, followed thereafter by a suppression of activity lasting up to a few seconds. Strong stimuli exceeding 50 % of maximal stimulator output could also lead to an early suppression of activity during the first 100-200 ms, followed by stronger (rebound) facilitation. Early suppression and facilitation of activity may be related to a more or less direct stimulation of inhibitory and excitatory interneurons, probably with different thresholds. The late, long-lasting suppression is more likely to be related to metabotropic or metabolic processes, or even vascular responses. The time course of facilitation/inhibition may provide clues regarding the action of repetitive TMS application.
Figures



References
-
- Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. - PubMed
-
- Amassian VE, Steward M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. - PubMed
-
- Baker SN, Olivier E, Lemon RN. Recording an identified pyramidal volley evoked by transcranial magnetic stimulation in a conscious macaque monkey. Exp Brain Res. 1994;99:529–532. - PubMed
-
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. - PubMed
-
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

