Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle
- PMID: 12963792
- PMCID: PMC2343481
- DOI: 10.1113/jphysiol.2003.049759
Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle
Abstract
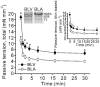
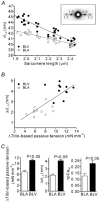
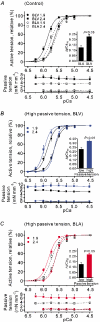
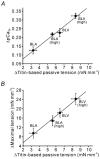
We have explored the role of the giant elastic protein titin in the Frank-Starling mechanism of the heart by measuring the sarcomere length (SL) dependence of activation in skinned cardiac muscles with different titin-based passive stiffness characteristics. We studied muscle from the bovine left ventricle (BLV), which expresses a high level of a stiff titin isoform, and muscle from the bovine left atrium (BLA), which expresses more compliant titin isoforms. Passive tension was also varied in each muscle type by manipulating the pre-history of stretch prior to activation. We found that the SL-dependent increases in Ca2+ sensitivity and maximal Ca2+-activated tension were markedly more pronounced when titin-based passive tension was high. Small-angle X-ray diffraction experiments revealed that the SL dependence of reduction of interfilament lattice spacing is greater in BLV than in BLA and that the lattice spacing is coupled with titin-based passive tension. These results support the notion that titin-based passive tension promotes actomyosin interaction by reducing the lattice spacing. This work indicates that titin may be a factor involved in the Frank-Starling mechanism of the heart by promoting actomyosin interaction in response to stretch.
Figures

Similar articles
-
Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle.Pflugers Arch. 2005 Feb;449(5):449-57. doi: 10.1007/s00424-004-1354-6. Epub 2004 Nov 20. Pflugers Arch. 2005. PMID: 15688246
-
Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes.Circ Res. 2001 May 25;88(10):1028-35. doi: 10.1161/hh1001.090876. Circ Res. 2001. PMID: 11375272
-
Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart.Circulation. 2001 Oct 2;104(14):1639-45. doi: 10.1161/hc3901.095898. Circulation. 2001. PMID: 11581142
-
Titin/connectin-based modulation of the Frank-Starling mechanism of the heart.J Muscle Res Cell Motil. 2005;26(6-8):319-23. doi: 10.1007/s10974-005-9038-1. J Muscle Res Cell Motil. 2005. PMID: 16453158 Review.
-
Role of the giant elastic protein titin in the Frank-Starling mechanism of the heart.Curr Vasc Pharmacol. 2004 Apr;2(2):135-9. doi: 10.2174/1570161043476357. Curr Vasc Pharmacol. 2004. PMID: 15320514 Review.
Cited by
-
Impact of titin isoform on length dependent activation and cross-bridge cycling kinetics in rat skeletal muscle.Biochim Biophys Acta. 2013 Apr;1833(4):804-11. doi: 10.1016/j.bbamcr.2012.08.011. Epub 2012 Aug 23. Biochim Biophys Acta. 2013. PMID: 22951219 Free PMC article.
-
Functional and structural differences between skinned and intact muscle preparations.J Gen Physiol. 2022 Feb 7;154(2):e202112990. doi: 10.1085/jgp.202112990. Epub 2022 Jan 19. J Gen Physiol. 2022. PMID: 35045156 Free PMC article. Review.
-
Myofilament length dependent activation.J Mol Cell Cardiol. 2010 May;48(5):851-8. doi: 10.1016/j.yjmcc.2009.12.017. Epub 2010 Jan 4. J Mol Cell Cardiol. 2010. PMID: 20053351 Free PMC article. Review.
-
Differences in Mechanical, Electrical and Calcium Transient Performance of the Isolated Right Atrial and Ventricular Myocardium of Guinea Pigs at Different Preloads (Lengths).Int J Mol Sci. 2023 Oct 24;24(21):15524. doi: 10.3390/ijms242115524. Int J Mol Sci. 2023. PMID: 37958508 Free PMC article.
-
Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling.Biophys J. 2004 Sep;87(3):1784-94. doi: 10.1529/biophysj.103.039131. Biophys J. 2004. PMID: 15345557 Free PMC article.
References
-
- Akella AB, Ding XL, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circ Res. 1995;76:600–606. - PubMed
-
- Allen DG, Kentish JC. The cellular basis for length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985;17:821–840. - PubMed
-
- Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. - PubMed
-
- Cazorla O, Vassort G, Garnier D, Le Guennec JY. Length modulation of active force in rat cardiac myocytes: is titin the sensor? J Mol Cell Cardiol. 1999;31:1215–1227. - PubMed
-
- Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001;88:1028–1035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

