Adenosine A(1) receptor-mediated presynaptic inhibition at the calyx of Held of immature rats
- PMID: 12963795
- PMCID: PMC2343556
- DOI: 10.1113/jphysiol.2003.048371
Adenosine A(1) receptor-mediated presynaptic inhibition at the calyx of Held of immature rats
Abstract
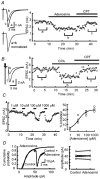
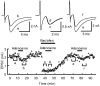
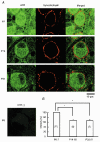
At the calyx of Held synapse in brainstem slices of 5- to 7-day-old (P5-7) rats, adenosine, or the type 1 adenosine (A1) receptor agonist N6-cyclopentyladenosine (CPA), inhibited excitatory postsynaptic currents (EPSCs) without affecting the amplitude of miniature EPSCs. The A1 receptor antagonist 8-cyclopentyltheophylline (CPT) had no effect on the amplitude of EPSCs evoked at a low frequency, but significantly reduced the magnitude of synaptic depression caused by repetitive stimulation at 10 Hz, suggesting that endogenous adenosine is involved in the regulation of transmitter release. Adenosine inhibited presynaptic Ca(2+) currents (IpCa) recorded directly from calyceal terminals, but had no effect on presynaptic K+ currents. When EPSCs were evoked by IpCa during simultaneous pre- and postsynaptic recordings, the magnitude of the adenosine-induced inhibition of IpCa fully explained that of EPSCs, suggesting that the presynaptic Ca(2+) channel is the main target of A1 receptors. Whereas the N-type Ca(2+) channel blocker omega-conotoxin attenuated EPSCs, it had no effect on the magnitude of adenosine-induced inhibition of EPSCs. During postnatal development, in parallel with a decrease in the A1 receptor immunoreactivity at the calyceal terminal, the inhibitory effect of adenosine became weaker. We conclude that presynaptic A1 receptors at the immature calyx of Held synapse play a regulatory role in transmitter release during high frequency transmission, by inhibiting multiple types of presynaptic Ca(2+) channels.
Figures


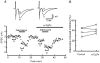



Similar articles
-
5-HT1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats.Eur J Neurosci. 2006 Oct;24(7):1946-54. doi: 10.1111/j.1460-9568.2006.05063.x. Eur J Neurosci. 2006. PMID: 17067296
-
Presynaptic inhibition at excitatory hippocampal synapses: development and role of presynaptic Ca2+ channels.J Neurophysiol. 1996 Jul;76(1):39-46. doi: 10.1152/jn.1996.76.1.39. J Neurophysiol. 1996. PMID: 8836207
-
Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats.J Physiol. 2001 Jun 1;533(Pt 2):423-31. doi: 10.1111/j.1469-7793.2001.0423a.x. J Physiol. 2001. PMID: 11389202 Free PMC article.
-
Neurosteroid pregnenolone sulfate enhances glutamatergic synaptic transmission by facilitating presynaptic calcium currents at the calyx of Held of immature rats.Eur J Neurosci. 2006 Oct;24(7):1955-66. doi: 10.1111/j.1460-9568.2006.05080.x. Epub 2006 Oct 16. Eur J Neurosci. 2006. PMID: 17040476
-
The action is at the terminal.J Physiol. 1999 Nov 1;520 Pt 3(Pt 3):629. doi: 10.1111/j.1469-7793.1999.t01-1-00629.x. J Physiol. 1999. PMID: 10545129 Free PMC article. Review.
Cited by
-
Glycolysis selectively shapes the presynaptic action potential waveform.J Neurophysiol. 2016 Dec 1;116(6):2523-2540. doi: 10.1152/jn.00629.2016. Epub 2016 Sep 7. J Neurophysiol. 2016. PMID: 27605535 Free PMC article.
-
Thalamocortical dynamics of sleep: roles of purinergic neuromodulation.Semin Cell Dev Biol. 2011 Apr;22(2):245-51. doi: 10.1016/j.semcdb.2011.02.008. Epub 2011 Feb 15. Semin Cell Dev Biol. 2011. PMID: 21329763 Free PMC article. Review.
-
Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels.J Physiol. 2007 Nov 1;584(Pt 3):835-51. doi: 10.1113/jphysiol.2007.139683. Epub 2007 Sep 6. J Physiol. 2007. PMID: 17823210 Free PMC article.
-
D1-like dopamine receptors selectively block P/Q-type calcium channels to reduce glutamate release onto cholinergic basal forebrain neurones of immature rats.J Physiol. 2007 Apr 1;580(Pt 1):103-17. doi: 10.1113/jphysiol.2006.125724. Epub 2007 Jan 18. J Physiol. 2007. PMID: 17234695 Free PMC article.
-
Purinergic Modulation of Activity in the Developing Auditory Pathway.Neurosci Bull. 2020 Nov;36(11):1285-1298. doi: 10.1007/s12264-020-00586-4. Epub 2020 Oct 11. Neurosci Bull. 2020. PMID: 33040238 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

