Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides
- PMID: 12965898
- PMCID: PMC193885
- DOI: 10.1128/cdli.10.5.744-750.2003
Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides
Abstract
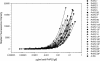
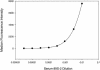
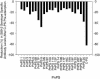
We describe a fluorescent covalent microsphere immunoassay (FCMIA) method for the simultaneous (multiplexed) measurement of immunoglobulin G (IgG) antibodies to 23 pneumococcal capsular polysaccharide (PnPS) serotypes present in the pneumococcal polysaccharide vaccine (PPV23) licensed by the Food and Drug Administration, i.e., PnPSs 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. In addition, the assay incorporates an internal control that allows for contemporaneous evaluation of the effectiveness of pneumococcal cell wall polysaccharide (C-PS) preadsorption and a second control of PnPS 25 (which is not present in any polysaccharide or conjugate vaccine), which can be used to evaluate interassay reproducibility (useful for pre- versus postvaccination studies). The FCMIA was standardized with U.S. reference antipneumococcal serotype standard serum 89S-2. Preadsorption of 89S-2 with each PnPS and C-PS yielded homologous inhibition for serotypes 1, 6B, 9N, 9V, 11A, 12F,14, 15B, 18C, 19A, 19F, 20, 22F, 25, and 33F; heterologous inhibition for serotypes 9V, 10A, 11A, 12F, 15B, 17F, 20, and 23F; and neither homologous nor heterologous inhibition for serotypes 2, 3, 4, and 5. The minimum detectable concentrations for the 24 multiplexed (PnPS and C-PS) FCMIAs ranged from 20 pg/ml for PnPS 3 to 600 pg/ml for PnPS 14. The PnPS FCMIA method has numerous benefits over enzyme-linked immunosorbent assays commonly used to measure anti-PnPS-specific IgG levels, including increased speed, smaller sample volumes, equivalent or better sensitivity, and increased dynamic range.
Figures

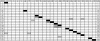
References
-
- Bellisario, R., R. J. Colinas, and K. A. Pass. 2000. Simultaneous measurement of thyroxine and thyrotropin from newborn dried blood-spot specimens using a multiplexed fluorescent microsphere immunoassay. Clin. Chem. 46:1422-1424. - PubMed
-
- Biagini, R. E., D. M. Murphy, D. L. Sammons, J. P. Smith, C. A. Striley, and B. A. MacKenzie. 2002. Development of multiplexed fluorescence microbead covalent assays (FMCAs) for pesticide biomonitoring. Bull. Environ. Contam. Toxicol. 68:470-477. - PubMed
-
- Centers for Disease Control and Prevention. 2002. Epidemiology and prevention of vaccine-preventable diseases, 7th ed. U.S. Department of Health and Human Services, Atlanta, Ga.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

