Detection of West Nile virus (WNV)-specific immunoglobulin M in a reference laboratory setting during the 2002 WNV season in the United States
- PMID: 12965901
- PMCID: PMC193906
- DOI: 10.1128/cdli.10.5.764-768.2003
Detection of West Nile virus (WNV)-specific immunoglobulin M in a reference laboratory setting during the 2002 WNV season in the United States
Abstract
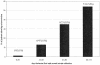
Between 1 June and 31 December 2002, 30,677 serum samples and 4,554 cerebrospinal fluid (CSF) samples were tested for West Nile virus (WNV)-specific immunoglobulin M (IgM) by an in-house enzyme-linked immunosorbent assay (ELISA); 1,481 serum samples (4.8%) and 345 CSF samples (7.6%) were positive for WNV IgM. Positive samples were forwarded to public health service laboratories (PHSLs) for further testing. PHSLs supplied results from their WNV IgM ELISAs for 654 samples; 633 (97%) were positive. PHSLs supplied WNV plaque reduction neutralization test results for 128 samples; 123 (96%) were positive. WNV IgM seroconversion and seroreversion trends were evaluated for 749 patients who each provided two serum samples that were tested during the study period. Of 574 patients whose first serum sample was IgM negative, 41 (7%) seroconverted (the second serum sample was IgM positive); of 175 patients whose first serum sample was IgM positive, 22 (13%) seroreverted (the second serum sample was IgM negative). The seroreversion rate was directly proportional to the time between serum sample collection; whereas only 1% of patients whose sera were collected <20 days apart showed seroreversion, 54% of patients whose sera were collected >60 days apart showed seroreversion. Conversion and reversion trends for CSF were evaluated for 68 patients. Of 54 patients whose first CSF specimen was IgM negative, 9 (17%) converted; none of 14 patients whose first CSF specimen was IgM positive reverted. Concomitant detection of WNV IgM in serum and CSF was assessed for 1,188 patients for whom paired serum and CSF specimens were available; for all 130 patients for whom IgM was detectable in CSF, IgM was also detectable in serum. These findings show that an in-house WNV IgM ELISA accurately identifies patients with WNV infection, document WNV IgM conversion and reversion trends, and demonstrate that WNV IgM detection in CSF is accompanied by WNV IgM detection in serum.
Figures
References
-
- Briese, T., X.-Y. Jia, C. Huang, L. J. Grady, and W. I. Lipkin. 1999. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet 354:1261-1262. - PubMed
-
- Centers for Disease Control and Prevention. 2002. West Nile Virus Activity—United States, 2001. Morb. Mortal. Wkly. Rep. 51:497-501. - PubMed
-
- Centers for Disease Control and Prevention. 2003. West Nile Virus update current case count. [Online.] http://www.cdc.gov/od/oc/media/wncount.htm. Accessed 21 April 2003.
-
- Davis, B. S., C.-J. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. - PMC - PubMed
-
- Marfin, A. A., and D. J. Gubler. 2001. West Nile encephalitis: an emerging disease in the United States. Clin. Infect. Dis. 33:1713-1719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

