Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England
- PMID: 12965904
- PMCID: PMC193909
- DOI: 10.1128/cdli.10.5.780-786.2003
Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England
Abstract
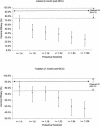
Meningococcal C conjugate (MCC) vaccines were licensed on the basis of serological correlates of protection without efficacy data. The original correlate of protection was established by using a serum bactericidal antibody assay (SBA) with human complement (hSBA), with titers > or =4 predicting protection. However, the antibody data supporting licensure were largely generated by SBA with rabbit complement (rSBA), which gives higher titers than hSBA. While rSBA titers > or =128 reliably predict protection, as measured by hSBA, sera with rSBA titers in the range of 8 to 64 may not have hSBA titers > or =4. For rSBA titers in this equivocal range, a fourfold rise pre- to postvaccination with the MCC vaccine and/or a characteristic booster response to a polysaccharide challenge was proposed as a correlate of protection. To validate this proposed rSBA correlate, age-specific efficacy estimates for MCC vaccines obtained from postlicensure surveillance in England were compared with the efficacy predicted by the percentage of individuals in these age groups with rSBA titers above different cutoffs at 4 weeks and at 7 to 9 months after vaccination with the MCC vaccine. The average time since vaccination in the cohorts in whom efficacy was measured ranged from 8 to 10 months. The rSBA cutoff of > or =128 was shown to significantly underestimate efficacy, with rSBA cutoffs of > or =4 or > or =8 at 4 weeks postvaccination with the MCC vaccine being the most consistent with observed efficacy. When the levels obtained 7 to 9 months postvaccination with the MCC vaccine were used, all rSBA cutoffs significantly underestimated efficacy, suggesting that continuing protection is less dependent on the SBA level at the time of exposure but is more reliant on immunologic memory.
Figures


References
-
- Biselli, R., A. Fattorossi, P. M. Matricardi, R. Nisini, T. Stroffolini, and R. d'Amelio. 1993. Dramatic reduction of meningococcal meningitis among military recruits in Italy after introduction of specific vaccination. Vaccine 11:578-581. - PubMed
-
- Borrow, R., and G. M. Carlone. 2001. Serogroup B and C serum bactericidal assays, p. 289-304. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines. Methods in molecular medicine. Humana Press, Totowa, N.J. - PubMed
-
- Burrage, M., A. Robinson, R. Borrow, N. Andrews, J. Southern, J. Findlow, S. Martin, C. Thornton, D. Goldblatt, M. Corbel, D. Sesardic, K. Cartwright, P. Richmond, and E. Miller. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946-4954. - PMC - PubMed
-
- De Wals, P., M. Dionne, M. Douville-Fradet, N. Boulianne, J. Drapeau, and G. de Serres. 2001. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Quebec. JAMA 285:177-181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

