Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients
- PMID: 12965921
- PMCID: PMC193887
- DOI: 10.1128/cdli.10.5.882-885.2003
Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients
Abstract
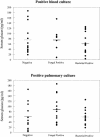
Fungal infections in the critically ill patient are difficult to diagnose and are associated with a high mortality rate. A major obstacle to managing fungal infection is the lack of a reliable clinical assay that will rapidly identify patients with fungal sepsis. Glucans are polymers of glucose that are found in the cell wall of fungi and certain bacteria. Glucans are also released from the fungal cell wall into the extracellular milieu. Several studies have reported that detection of fungal glucan in serum or plasma is useful in the diagnosis of mycoses. However, recent studies have questioned the clinical utility of this assay. In this study, we examined serum glucan levels in intensive care unit (ICU) patients and attempt to correlate serum glucan levels with the presence of fungal infection. Following attainment of informed consent, serum was harvested from 46 ICU patients with confirmed fungal infections, confirmed bacterial infections, or no evidence of infection. Sera from eight healthy volunteers served as control. Serum glucan was assayed with a glucan-specific Limulus assay. Serum glucan levels were increased (69.6 +/- 17 pg/ml; P < 0.001) in ICU patients versus the normal (11.5 +/- 1.3 pg/ml) and noninfected ICU (27.4 +/- 17 pg/ml) controls. However, serum glucan levels were not different in patients with confirmed fungal infections versus those with confirmed bacterial infections. Thus, serum glucan levels did not show a correlation with the presence of fungal infections and do not appear to be specific for fungal infections. However, the assay may be useful as a negative predictor of infection.
Figures

Similar articles
-
At low serum glucan concentrations there is an inverse correlation between serum glucan and serum cytokine levels in ICU patients with infections.Int Immunopharmacol. 2004 Aug;4(8):1107-15. doi: 10.1016/j.intimp.2004.05.010. Int Immunopharmacol. 2004. PMID: 15222986
-
Invasive fungal infections and (1,3)-beta-D-glucan serum concentrations in long-term intensive care patients.Int J Infect Dis. 2009 Nov;13(6):707-12. doi: 10.1016/j.ijid.2008.10.013. Epub 2009 Jan 20. Int J Infect Dis. 2009. PMID: 19157947
-
A new method for the quantification of beta-glucan in plasma and its application in the diagnosis of postoperative infection.Jpn J Surg. 1990 Sep;20(5):559-66. doi: 10.1007/BF02471013. Jpn J Surg. 1990. PMID: 2123014
-
[Plasma (1-->3)-beta-D-glucan determination for screening deep mycosis].Rinsho Byori. 1996 Jun;44(6):528-32. Rinsho Byori. 1996. PMID: 8752730 Review. Japanese.
-
Application of the 1,3-β-D-Glucan (Fungitell) Assay in the Diagnosis of Invasive Fungal Infections.Arch Pathol Lab Med. 2016 Feb;140(2):181-5. doi: 10.5858/arpa.2014-0230-RS. Arch Pathol Lab Med. 2016. PMID: 26910223 Review.
Cited by
-
How to interpret serum levels of beta-glucan for the diagnosis of invasive fungal infections in adult high-risk hematology patients: optimal cut-off levels and confounding factors.Eur J Clin Microbiol Infect Dis. 2015 May;34(5):917-25. doi: 10.1007/s10096-014-2302-9. Epub 2015 Jan 9. Eur J Clin Microbiol Infect Dis. 2015. PMID: 25573536
-
Invasive aspergillosis in the ICU: an emerging disease.Intensive Care Med. 2007 Oct;33(10):1679-81. doi: 10.1007/s00134-007-0792-y. Epub 2007 Jul 24. Intensive Care Med. 2007. PMID: 17646965 No abstract available.
-
Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1-->3)-beta-D-glucan antigens.Eur J Clin Microbiol Infect Dis. 2007 Nov;26(11):755-66. doi: 10.1007/s10096-007-0373-6. Eur J Clin Microbiol Infect Dis. 2007. PMID: 17671803 Review.
-
Dectin-1 multimerization and signaling depends on fungal β-glucan structure and exposure.Biophys J. 2023 Sep 19;122(18):3749-3767. doi: 10.1016/j.bpj.2023.07.021. Epub 2023 Jul 27. Biophys J. 2023. PMID: 37515324 Free PMC article.
-
Reactivity of (1→3)-β-d-glucan assay in bacterial bloodstream infections.Eur J Clin Microbiol Infect Dis. 2011 Nov;30(11):1453-60. doi: 10.1007/s10096-011-1244-8. Epub 2011 Apr 10. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21479838
References
-
- Hiyoshi, M., S. Tagawa, S. Hashimoto, C. Sakamoto, and N. Tatsumi. 1999. Evaluation of a new laboratory test measuring plasma (1→3)-β-D-glucan in the diagnosis of Candida deep mycosis: comparison with a serologic test. Kansenshogaku Zasshi 73:1-6. - PubMed
-
- Iwama, A., M. Yoshida, A. Miwa, T. Obayashi, S. Sakamoto, and Y. Miura. 1993. Improved survival from fungaemia in patients with haematological malignancies: analysis of risk factors for death and usefulness of early antifungal therapy. Eur. J. Haematol. 51:156-160. - PubMed
-
- Kami, M., Y. Tanaka, Y. Kanda, S. Ogawa, T. Masumoto, K. Ohtomo, T. Matsumura, T. Saito, U. Machida, T. Kashima, and H. Hirai. 2000. Computer tomographic scan of the chest, latex agglutination test and plasma (1→3)-β-D-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745-752. - PubMed
-
- Kohno, S., K. Mitsutake, S. Maesaki, A. Yasuoka, T. Miyazaki, M. Kaku, H. Koga, and Hara K. 1993. An evaluation of serodiagnostic tests in patients with candidemia: beta-glucan, mannan, candida antigen by Cand-Tec and D-arabinitol. Microbiol. Immunol. 37:207-212. - PubMed
-
- Mori, T., H. Ikemoto, M. Matsumura, M. Yoshida, K. Inada, S. Endo, A. Ito, S. Watanabe, H. Yamaguchi, M. Mitsuya, M. Kodama, T. Tani, T. Yokota, T. Kobayashi, J. Kambayashi, T. Nakamura, T. Masaoka, H. Teshima, T. Yoshinaga, S. Kohno, K. Hara, and S. Miyazaki. 1997. Evaluation of plasma (1→3)-beta-D-glucan measurement by the kinetic turbidimetric Limulus test, for the clinical diagnosis of mycotic infections. Eur. J. Clin. Chem. Biochem. 35:553-560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

