Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic Naegleria fowleri
- PMID: 12965933
- PMCID: PMC193893
- DOI: 10.1128/cdli.10.5.954-959.2003
Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic Naegleria fowleri
Abstract
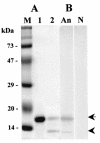
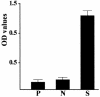
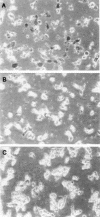
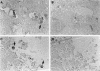
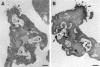
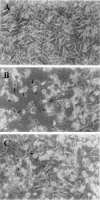
We previously cloned an antigenic gene (named nfa1) from a cDNA library of Naegleria fowleri by immunoscreening. The nfa1 gene had a coding nucleotide sequence consisting of 357 bases and produced a recombinant 13.1-kDa protein (Nfa1). In this study, to get more information regarding the recombinant Nfa1 protein (rNfa1), we produced an anti-Nfa1 polyclonal antibody from mice immunized with rNfa1 and used a peroxidase staining method to carry out immunocytochemistry experiments. In addition, we observed the effect of the presence of an anti-Nfa1 antibody on the in vitro cytotoxicity of N. fowleri against Chinese hamster ovary (CHO) cells. Trophozoites of N. fowleri in cultivation reacted strongly with a peroxidase-labeled anti-Nfa1 antibody. In inflammatory and necrotic regions of brain tissue infected with N. fowleri, labeled trophozoites that were stained brown were also observed. When examined using a transmission electron microscope, the Nfa1 protein showed pseudopodium-specific immunolocalization on a trophozoite of N. fowleri. When examined using a light microscope, CHO cells grown in cocultures with N. fowleri trophozoites (group I) for 48 h showed morphologically severe destruction but CHO cells grown in cocultures with N. fowleri trophozoites and an anti-Nfa1 polyclonal antibody (group II) showed less destruction. The results of a lactate dehydrogenase release assay showed that group I CHO cells exhibited 81% cytotoxicity and group II CHO cells exhibited 13.8% cytotoxicity.
Figures
Similar articles
-
Role of the Nfa1 protein in pathogenic Naegleria fowleri cocultured with CHO target cells.Clin Diagn Lab Immunol. 2005 Jul;12(7):873-6. doi: 10.1128/CDLI.12.7.873-876.2005. Clin Diagn Lab Immunol. 2005. PMID: 16002638 Free PMC article.
-
Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria fowleri.Korean J Parasitol. 2004 Mar;42(1):35-40. doi: 10.3347/kjp.2004.42.1.35. Korean J Parasitol. 2004. PMID: 15060338 Free PMC article.
-
Production of Nfa1-specific monoclonal antibodies that influences the in vitro cytotoxicity of Naegleria fowleri trophozoites on microglial cells.Parasitol Res. 2007 Oct;101(5):1191-6. doi: 10.1007/s00436-007-0600-1. Epub 2007 Jul 4. Parasitol Res. 2007. PMID: 17610083
-
Understanding the pathogenicity of Naegleria fowleri in association with N. fowleri antigen-1 (Nfa1).Parasites Hosts Dis. 2024 Nov;62(4):385-398. doi: 10.3347/PHD.24025. Epub 2024 Nov 22. Parasites Hosts Dis. 2024. PMID: 39622651 Free PMC article. Review.
-
The immune response to Naegleria fowleri amebae and pathogenesis of infection.FEMS Immunol Med Microbiol. 2007 Nov;51(2):243-59. doi: 10.1111/j.1574-695X.2007.00332.x. Epub 2007 Sep 21. FEMS Immunol Med Microbiol. 2007. PMID: 17894804 Review.
Cited by
-
Role of the Nfa1 protein in pathogenic Naegleria fowleri cocultured with CHO target cells.Clin Diagn Lab Immunol. 2005 Jul;12(7):873-6. doi: 10.1128/CDLI.12.7.873-876.2005. Clin Diagn Lab Immunol. 2005. PMID: 16002638 Free PMC article.
-
The immune response induced by DNA vaccine expressing nfa1 gene against Naegleria fowleri.Parasitol Res. 2012 Dec;111(6):2377-84. doi: 10.1007/s00436-012-3093-5. Epub 2012 Sep 7. Parasitol Res. 2012. PMID: 22955499
-
Vaccination with lentiviral vector expressing the nfa1 gene confers a protective immune response to mice infected with Naegleria fowleri.Clin Vaccine Immunol. 2013 Jul;20(7):1055-60. doi: 10.1128/CVI.00210-13. Epub 2013 May 15. Clin Vaccine Immunol. 2013. PMID: 23677321 Free PMC article.
-
Protective immunity against Naegleria fowleri infection on mice immunized with the rNfa1 protein using mucosal adjuvants.Parasitol Res. 2015 Apr;114(4):1377-85. doi: 10.1007/s00436-015-4316-3. Epub 2015 Jan 22. Parasitol Res. 2015. PMID: 25604672
-
Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria fowleri.Korean J Parasitol. 2004 Mar;42(1):35-40. doi: 10.3347/kjp.2004.42.1.35. Korean J Parasitol. 2004. PMID: 15060338 Free PMC article.
References
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Carter, R. F. 1970. Description of a Naegleria species isolated from two cases of primary amoebic meningoencephalitis, and of the experimental pathological changes induced by it. J. Pathol. 100:217-244. - PubMed
-
- Cline, M., R. Catchman, and F. Marciano-Cabral. 1986. Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J. Protozool. 33:10-13. - PubMed
-
- Culbertson, C. G. 1971. The pathogenicity of soil amoebas. Annu. Rev. Microbiol. 25:231-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

