Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification
- PMID: 12966144
- PMCID: PMC193871
- DOI: 10.1128/MMBR.67.3.454-472.2003
Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification
Abstract
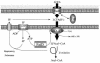
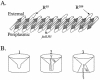
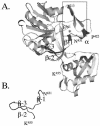
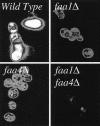
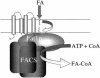
The processes that govern the regulated transport of long-chain fatty acids across the plasma membrane are quite distinct compared to counterparts involved in the transport of hydrophilic solutes such as sugars and amino acids. These differences stem from the unique physical and chemical properties of long-chain fatty acids. To date, several distinct classes of proteins have been shown to participate in the transport of exogenous long-chain fatty acids across the membrane. More recent work is consistent with the hypothesis that in addition to the role played by proteins in this process, there is a diffusional component which must also be considered. Central to the development of this hypothesis are the appropriate experimental systems, which can be manipulated using the tools of molecular genetics. Escherichia coli and Saccharomyces cerevisiae are ideally suited as model systems to study this process in that both (i) exhibit saturable long-chain fatty acid transport at low ligand concentrations, (ii) have specific membrane-bound and membrane-associated proteins that are components of the transport apparatus, and (iii) can be easily manipulated using the tools of molecular genetics. In both systems, central players in the process of fatty acid transport are fatty acid transport proteins (FadL or Fat1p) and fatty acyl coenzyme A (CoA) synthetase (FACS; fatty acid CoA ligase [AMP forming] [EC 6.2.1.3]). FACS appears to function in concert with FadL (bacteria) or Fat1p (yeast) in the conversion of the free fatty acid to CoA thioesters concomitant with transport, thereby rendering this process unidirectional. This process of trapping transported fatty acids represents one fundamental mechanism operational in the transport of exogenous fatty acids.
Figures
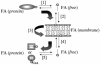





References
-
- Abumrad, N. A., R. C. Perkins, J. H. Park, and C. R. Park. 1981. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J. Biol. Chem. 256:9183-9191. - PubMed
-
- Abumrad, N. A., J. H. Park, and C. R. Park. 1984. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J. Biol. Chem. 259:8945-8953. - PubMed
-
- Abumrad, N., C. Harmon, and A. Ibrahimi. 1998. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J. Lipid Res. 39:2309-2318. - PubMed
-
- Abumrad, N. A., M. R. el-Maghrabi, E. Z. Amri, E. Lopez, and P. A. Grimaldi. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268:17665-17668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

