Heat shock cognate protein 70 encodes antigenic epitopes recognised by HLA-B4601-restricted cytotoxic T lymphocytes from cancer patients
- PMID: 12966429
- PMCID: PMC2376957
- DOI: 10.1038/sj.bjc.6601203
Heat shock cognate protein 70 encodes antigenic epitopes recognised by HLA-B4601-restricted cytotoxic T lymphocytes from cancer patients
Abstract
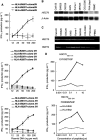
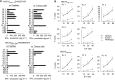
Heat shock cognate protein 70 (HSC70), a highly conserved protein and a member of the family of molecular chaperones, has the ability to induce cytotoxic T lymphocyte (CTL) responses through binding and carrying antigenic peptides. We demonstrated in this study that the HSC70 gene encodes two antigenic peptides recognised by HLA-B46-restricted and tumour-reactive CTLs established from tumour-infiltrating lymphocytes of a colon cancer. These HSC70-derived peptides, at amino-acid positions 106-114 and 233-241, had the ability to induce HLA-B46-restricted and peptide-specific CTLs, which are reactive to tumour cells, from peripheral blood mononuclear cells of the majority of epithelial cancer patients tested. These results, along with those from the previous studies, indicate the two ways of HSC70 involvement in the immune responses to tumours: chaperones and antigens, and thus may provide a new insight for the development of HSC70-directed cancer-specific immunotherapy.
Figures

Similar articles
-
Mutated p53 gene encodes a nonmutated epitope recognized by HLA-B*4601-restricted and tumor cell-reactive CTLs at tumor site.Cancer Res. 2003 Feb 15;63(4):854-8. Cancer Res. 2003. PMID: 12591737
-
A cyclophilin B gene encodes antigenic epitopes recognized by HLA-A24-restricted and tumor-specific CTLs.J Immunol. 1999 Nov 1;163(9):4994-5004. J Immunol. 1999. PMID: 10528204
-
Two proliferation-related proteins, TYMS and PGK1, could be new cytotoxic T lymphocyte-directed tumor-associated antigens of HLA-A2+ colon cancer.Clin Cancer Res. 2004 Sep 1;10(17):5828-36. doi: 10.1158/1078-0432.CCR-04-0350. Clin Cancer Res. 2004. PMID: 15355913
-
Cytotoxic T cell responses against immunoglobulin in malignant and normal B cells: implications for tumor immunity and autoimmunity.Curr Pharm Des. 2003;9(23):1889-903. doi: 10.2174/1381612033454333. Curr Pharm Des. 2003. PMID: 12871193 Review.
-
Activation of cytotoxic T cells by solid tumours?Cell Mol Life Sci. 1998 Mar;54(3):263-71. doi: 10.1007/s000180050148. Cell Mol Life Sci. 1998. PMID: 9575338 Free PMC article. Review.
Cited by
-
Single-Cell Sequencing to Identify Six Heat Shock Protein (HSP) Genes-Mediated Progression Subtypes of Clear Cell Renal Cell Carcinoma.Int J Gen Med. 2021 Jul 23;14:3761-3773. doi: 10.2147/IJGM.S318271. eCollection 2021. Int J Gen Med. 2021. PMID: 34326662 Free PMC article.
-
HSP70s: From Tumor Transformation to Cancer Therapy.Clin Med Oncol. 2008;2:335-45. doi: 10.4137/cmo.s475. Epub 2008 Apr 24. Clin Med Oncol. 2008. PMID: 21892295 Free PMC article.
-
Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa.Cell Stress Chaperones. 2011 May;16(3):235-49. doi: 10.1007/s12192-010-0236-4. Epub 2010 Oct 21. Cell Stress Chaperones. 2011. PMID: 20963644 Free PMC article. Review.
-
GRP-induced up-regulation of Hsp72 promotes CD16+/94+ natural killer cell binding to colon cancer cells causing tumor cell cytolysis.Clin Exp Metastasis. 2008;25(4):451-63. doi: 10.1007/s10585-008-9151-9. Epub 2008 Mar 19. Clin Exp Metastasis. 2008. PMID: 18350254
-
Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe?Cell Stress Chaperones. 2006 Spring;11(1):1-12. doi: 10.1379/csc-155r.1. Cell Stress Chaperones. 2006. PMID: 16572724 Free PMC article. Review.
References
-
- Akesaka T, Kashiwase K, Shimamura M, Ishikawa Y, Tanaka H, Fujii M, Akaza T, Hando K, Yuasa S, Takahashi T, Juji T (2000) Identification of a novel HLA-B46 allele, B*4602, in Japanese. Tissue Antigens 55: 460–462 - PubMed
-
- Azuma K, Shichijo S, Maeda Y, Nakatsura T, Nonaka Y, Fujii T, Koike K, Itoh K (2003) Mutated p53 gene encodes a non-mutated epitope recognized by HLA-B4601-restricted and tumor cell-reactive CTLs at tumor site. Cancer Res 63: 854–858 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

