Potentiation of tumour apoptosis by human growth hormone via glutathione production and decreased NF-kappaB activity
- PMID: 12966434
- PMCID: PMC2376966
- DOI: 10.1038/sj.bjc.6601223
Potentiation of tumour apoptosis by human growth hormone via glutathione production and decreased NF-kappaB activity
Abstract
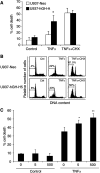
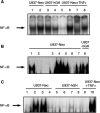
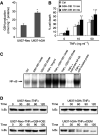
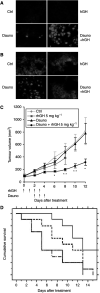
In addition to its primary role as growth factor, human growth hormone (hGH) can also participate in cell survival, as already documented by its protective effect on human monocytes or human promyelocytic leukaemia U937 cells exposed to a Fas-mediated cell death signal. However, despite similarities in the molecular events following Fas and TNF-alpha receptor engagement, we report that U937 cells, genetically engineered to constitutively produce hGH, were made more sensitive to TNF-alpha-induced apoptosis than parental cells. This was due to overproduction of the antioxidant glutathione, which decreased the nuclear factor (NF)-kappaB activity known to control the expression of survival genes. These findings were confirmed in vivo, in nude mice bearing U937 tumours coinjected with recombinant hGH and the NF-kappaB -inducing anticancer drug daunorubicin, to avoid the in vivo toxicity of TNF-alpha. This study therefore highlights one of the various properties of hGH that may have potential clinical implications.
Figures

Similar articles
-
Regulation of Fas (CD95)-induced apoptosis by nuclear factor-kappaB and tumor necrosis factor-alpha in macrophages.Am J Physiol Cell Physiol. 2002 Sep;283(3):C831-8. doi: 10.1152/ajpcell.00045.2002. Am J Physiol Cell Physiol. 2002. PMID: 12176740
-
Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes.Clin Cancer Res. 2004 May 1;10(9):3169-78. doi: 10.1158/1078-0432.ccr-03-0586. Clin Cancer Res. 2004. PMID: 15131058
-
Inhibition of ICAM-1 gene expression, monocyte adhesion and cancer cell invasion by targeting IKK complex: molecular and functional study of novel alpha-methylene-gamma-butyrolactone derivatives.Carcinogenesis. 2004 Oct;25(10):1925-34. doi: 10.1093/carcin/bgh211. Epub 2004 Jun 24. Carcinogenesis. 2004. PMID: 15217903
-
Human growth hormone gene transfer into tumor cells may improve cancer chemotherapy.Cancer Gene Ther. 2002 Jun;9(6):497-504. doi: 10.1038/sj.cgt.7700467. Cancer Gene Ther. 2002. PMID: 12032660
-
Combined treatment with verrucarin A and tumor necrosis factor-α sensitizes apoptosis by overexpression of nuclear factor-kappaB-mediated Fas.Environ Toxicol Pharmacol. 2013 Sep;36(2):303-310. doi: 10.1016/j.etap.2013.04.008. Epub 2013 Apr 25. Environ Toxicol Pharmacol. 2013. PMID: 23708311
Cited by
-
Genetic variants contributing to daunorubicin-induced cytotoxicity.Cancer Res. 2008 May 1;68(9):3161-8. doi: 10.1158/0008-5472.CAN-07-6381. Cancer Res. 2008. PMID: 18451141 Free PMC article.
-
The glutathione antioxidant system is enhanced in growth hormone transgenic coho salmon (Oncorhynchus kisutch).J Comp Physiol B. 2007 May;177(4):413-22. doi: 10.1007/s00360-006-0140-5. Epub 2007 Jan 16. J Comp Physiol B. 2007. PMID: 17225138
References
-
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C (1996) Mechanism of signaling by growth hormone receptor. Cell 76: 1089–1107 - PubMed
-
- Arlt A, Vorndamm J, Breitenbroich M, Folsch UR, Kalthoff H, Schmidt WE, Schafer H (2001) Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene 20: 859–868 - PubMed
-
- Baeuerle PA, Baltimore D (1988) IκB: a specific inhibitor of the NF-κB transcription factor. Science 242: 540–546 - PubMed
-
- Cherbonnier C, Déas O, Vassal G, Merlin J-L, Haeffner A, Senik A, Charpentier B, Dürrbach A, Bénard J, Hirsch F (2002) Human growth hormone gene transfer into tumor cells may improve cancer chemotherapy. Cancer Gene Ther 9: 497–504 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

