TNF-alpha increases human melanoma cell invasion and migration in vitro: the role of proteolytic enzymes
- PMID: 12966436
- PMCID: PMC2376936
- DOI: 10.1038/sj.bjc.6601257
TNF-alpha increases human melanoma cell invasion and migration in vitro: the role of proteolytic enzymes
Abstract
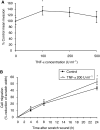
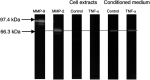
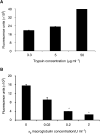
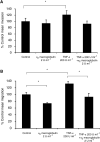
Inflammatory mediators have been reported to promote malignant cell growth, invasion and metastatic potential. More specifically, we have recently reported that tumour necrosis factor alpha (TNF-alpha) increases melanoma cell attachment to extracellular matrix (ECM) substrates and invasion through fibronectin. In this study, we extend these investigations asking specifically whether the TNF-alpha effect on cell invasion and migration involves activation of proteolytic enzymes. We examined the effect of TNF-alpha on melanoma expression/activation of type IV gelatinases matrix metalloproteinases 2 and 9 (MMPs -2 and -9) and general proteolytic enzymes. Stimulation with TNF-alpha significantly increased both melanoma cell migration at 24 h (+21%) and invasion through fibronectin (+35%) but did not upregulate/activate the expression of latent MMP-2 constitutively produced by these cells and did not upregulate their general protease activity. However, the increased cell migration and invasion through fibronectin observed following stimulation with TNF-alpha were inhibited by the general protease inhibitor alpha(2) macroglobulin. These findings suggest that the promigratory and proinvasive effect of TNF-alpha on this melanoma cell line may be mediated to some extent by induction of localised cell membrane-bound degradative enzyme activity, which is not readily detected in biochemical assays.
Figures
Similar articles
-
Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras.Melanoma Res. 2011 Aug;21(4):267-73. doi: 10.1097/CMR.0b013e3283414444. Melanoma Res. 2011. PMID: 21734530
-
Sodium salicylate inhibits TNF-alpha-induced NF-kappaB activation, cell migration, invasion and ICAM-1 expression in human melanoma cells.Melanoma Res. 2006 Feb;16(1):11-22. doi: 10.1097/01.cmr.0000195698.58013.53. Melanoma Res. 2006. PMID: 16432451
-
Melanoma invasion in reconstructed human skin is influenced by skin cells--investigation of the role of proteolytic enzymes.Clin Exp Metastasis. 2003;20(8):685-700. doi: 10.1023/b:clin.0000006824.41376.b0. Clin Exp Metastasis. 2003. PMID: 14713103
-
The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis.Pharmacol Res. 2016 Sep;111:17-22. doi: 10.1016/j.phrs.2016.05.019. Epub 2016 May 21. Pharmacol Res. 2016. PMID: 27221755 Review.
-
[Involvement of matrix metalloproteinases (MMPs) in cutaneous melanoma progression].Pathol Biol (Paris). 2004 Apr;52(3):154-9. doi: 10.1016/j.patbio.2004.02.002. Pathol Biol (Paris). 2004. PMID: 15063935 Review. French.
Cited by
-
TNF-α promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts.Cell Oncol (Dordr). 2016 Aug;39(4):353-63. doi: 10.1007/s13402-016-0280-x. Epub 2016 Apr 4. Cell Oncol (Dordr). 2016. PMID: 27042827 Free PMC article.
-
WBP2 promotes BTRC mRNA stability to drive migration and invasion in triple-negative breast cancer via NF-κB activation.Mol Oncol. 2022 Jan;16(2):422-446. doi: 10.1002/1878-0261.13048. Epub 2021 Aug 12. Mol Oncol. 2022. PMID: 34197030 Free PMC article.
-
C5aR1 regulates migration of suppressive myeloid cells required for costimulatory blockade-induced murine allograft survival.Am J Transplant. 2019 Mar;19(3):633-645. doi: 10.1111/ajt.15072. Epub 2018 Sep 17. Am J Transplant. 2019. PMID: 30106232 Free PMC article.
-
A comparative study of adhesion of melanoma and breast cancer cells to blood and lymphatic endothelium.Lymphat Res Biol. 2012 Dec;10(4):173-81. doi: 10.1089/lrb.2012.0007. Epub 2012 Dec 5. Lymphat Res Biol. 2012. PMID: 23215743 Free PMC article.
-
Mechanism of action differences in the antitumor effects of transmembrane and secretory tumor necrosis factor-alpha in vitro and in vivo.Cancer Immunol Immunother. 2006 Dec;55(12):1470-9. doi: 10.1007/s00262-006-0150-x. Epub 2006 Mar 23. Cancer Immunol Immunother. 2006. PMID: 16555058 Free PMC article.
References
-
- Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kähäri V-M (2003) Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene 22: 2121–2134 - PubMed
-
- Balkwill F (2002) Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 13: 135–141 - PubMed
-
- Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357: 539–545 - PubMed
-
- Baugh MD, Perry MJ, Hollander AP, Davies RD, Cross SS, Lobo AJ, Taylor CJ, Evans GS (1999) Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 117: 814–822 - PubMed
-
- Beutler BA (1999) The role of tumor necrosis factor in health and disease. J Rheumatol 26S(57): 16–21 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

