Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts
- PMID: 12967916
- PMCID: PMC2823795
- DOI: 10.1152/ajpcell.00428.2002
Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts
Abstract
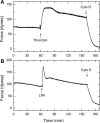
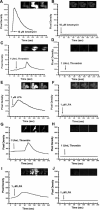
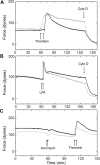
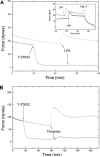
Thus far, determining the relative contribution of Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) and Ca2+-independent Rho-kinase pathways to myosin II activation and contraction has been difficult. In this study, we characterize the role of Rho-kinase in a rat embryo fibroblast cell line (REF-52), which contains no detectable MLCK. No endogenous MLCK could be detected in REF-52 cells by either Western or Northern blot analysis. In the presence or absence of Ca2+, thrombin or lysophosphatidic acid (LPA) increased RhoA activity and Rhokinase activity, correlating with isometric tension development and myosin II regulatory light chain (RLC) phosphorylation. Resting tension is associated with a basal phosphorylation of 0.31 +/- 0.02 mol PO4/mol RLC, whereas upon LPA or thrombin treatment myosin II RLC phosphorylation increases to 1.08 +/- 0.05 and 0.82 +/- 0.05 mol PO4/mol RLC, respectively, within 2.5 min. Ca2+ chelation has minimal effect on the kinetics and magnitude of isometric tension development and RLC phosphorylation. Treatment of REF-52 cells with the Rho-kinase-specific inhibitor Y-27632 abolished thrombin- and LPA-stimulated contraction and RLC phosphorylation. These results suggest that Rho-kinase is sufficient to activate myosin II motor activity and contraction in REF-52 cells.
Figures





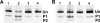
References
-
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Birukov KG, Schavocky JP, Shirinsky VP, Chibalina MV, Van Eldik LJ, Watterson DM. Organization of the genetic locus for chicken myosin light chain kinase is complex: multiple proteins are encoded and exhibit differential expression and localization. J Cell Biochem. 1998;70:402–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

