Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons
- PMID: 12967982
- PMCID: PMC6740684
- DOI: 10.1523/JNEUROSCI.23-23-08212.2003
Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons
Abstract
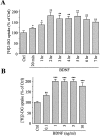
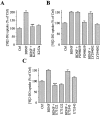
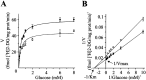
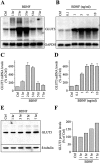
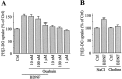
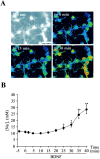
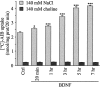
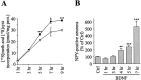
Brain-derived neurotrophic factor (BDNF) promotes the biochemical and morphological differentiation of selective populations of neurons during development. In this study we examined the energy requirements associated with the effects of BDNF on neuronal differentiation. Because glucose is the preferred energy substrate in the brain, the effect of BDNF on glucose utilization was investigated in developing cortical neurons via biochemical and imaging studies. Results revealed that BDNF increases glucose utilization and the expression of the neuronal glucose transporter GLUT3. Stimulation of glucose utilization by BDNF was shown to result from the activation of Na+/K+-ATPase via an increase in Na+ influx that is mediated, at least in part, by the stimulation of Na+-dependent amino acid transport. The increased Na+-dependent amino acid uptake by BDNF is followed by an enhancement of overall protein synthesis associated with the differentiation of cortical neurons. Together, these data demonstrate the ability of BDNF to stimulate glucose utilization in response to an enhanced energy demand resulting from increases in amino acid uptake and protein synthesis associated with the promotion of neuronal differentiation by BDNF.
Figures
References
-
- Ames 3rd A ( 2000) CNS energy metabolism as related to function. Brain Res Brain Res Rev 34 : 42-68. - PubMed
-
- Astrup J, Sorensen PM, Sorensen HR ( 1981) Oxygen and glucose consumption related to Na+/K+ transport in canine brain. Stroke 12 : 726-730. - PubMed
-
- Attwell D, Laughlin SB ( 2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21 : 1133-1145. - PubMed
-
- Barbacid M ( 1994) The Trk family of neurotrophin receptors. J Neurobiol 25 : 1386-1403. - PubMed
-
- Barnea A, Cho G, Lu G, Mathis M ( 1995) Brain-derived neurotrophic factor induces functional expression and phenotypic differentiation of cultured fetal neuropeptide Y-producing neurons. J Neurosci Res 42 : 638-647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
