Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development
- PMID: 12967983
- PMCID: PMC6740693
- DOI: 10.1523/JNEUROSCI.23-23-08221.2003
Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development
Abstract
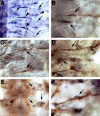
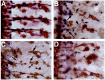
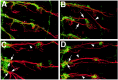
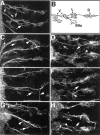
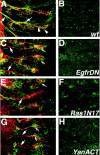
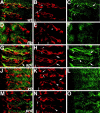
A major developmental role of peripheral glia is to mediate sensory axon guidance; however, it is not known whether sensory neurons influence peripheral glial development. To determine whether glia and neurons reciprocally interact during embryonic development, we ablated each cell type by overexpressing the apoptosis gene, grim, and observed the effects on peripheral nervous system (PNS) development. When neurons are ablated, glial defects occur as a secondary effect, and vice versa. Therefore glia and neurons are codependent during embryogenesis. To further explore glial-neuronal interactions, we genetically disrupted glial migration or differentiation and observed the secondary effects on sensory neuron development. Glial migration and ensheathment of PNS axons was blocked by overexpression of activated Rho GTPase, a regulator of actin dynamics. Here, sensory axons extended to the CNS without exhibiting gross pathfinding errors. In contrast, disrupting differentiation by expression of dominant-negative Ras GTPase in glia resulted in major sensory axon pathfinding errors, similar to those seen in glial ablations. Glial overexpression of transgenic components of the epidermal growth factor receptor (EGFR) signaling pathway yielded similar sensory neuron defects and also downregulated the expression of the glial marker Neuroglian. Mutant analysis also suggested that the EGFR ligands Spitz and Vein play roles in peripheral glial development. The observations support a model in which glia express genes necessary for sensory neuron development, and these genes are potentially under the control of the EGFR/Ras signaling pathway.
Figures

References
-
- Auld VJ, Fetter RD, Broadie K, Goodman CS ( 1995) Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila Cell 81 : 757-767. - PubMed
-
- Bergmann A, Tugentman M, Shilo B-Z, Steller H ( 2002) Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev Cell 2 : 159-170. - PubMed
-
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS ( 1989) Drosophila Neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell 59 : 447-460. - PubMed
-
- Booth GE, Kinrade EFV, Hidalgo A ( 2000) Glia maintain follower neuron survival during Drosophila CNS development. Development 127 : 237-244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
