Chromosomal loci influencing the susceptibility to the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PMID: 12967986
- PMCID: PMC6740699
- DOI: 10.1523/JNEUROSCI.23-23-08247.2003
Chromosomal loci influencing the susceptibility to the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Abstract
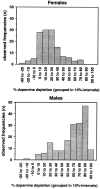
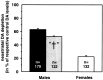
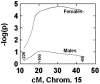
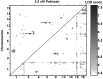
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the dysfunction of the nigrostriatal dopaminergic pathway. Although its etiology is not yet fully understood, an interaction of genetic predisposition and environmental factors is frequently discussed. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can evoke PD-like symptoms and neuropathological changes in various species, including mice. It was found repeatedly that mouse strains differ in their susceptibility to MPTP, which might serve as a model for genetic predisposition to neurodegeneration of the nigrostriatal system. In the present study, F2 intercross mice, derived from parental strains with high (C57BL/6J) versus low (BALB/cJ) MPTP susceptibility, were treated with MPTP and phenotyped for dopamine (DA) loss in the neostriatum, a highly sensitive marker of nigrostriatal dysfunction. A subsequent quantitative trait loci analysis revealed a gender-dependent locus for DA loss on chromosome 15 and a putative locus on chromosome 13. A number of potential candidate genes, including the membrane dopamine transporter, are located in the respective areas. Several mechanisms that are possibly involved in the control of the action of MPTP on the nigrostriatal system are discussed.
Figures

Similar articles
-
Temporal mRNA profiles of inflammatory mediators in the murine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine model of Parkinson's disease.Neuroscience. 2007 Mar 16;145(2):654-68. doi: 10.1016/j.neuroscience.2006.12.030. Epub 2007 Jan 29. Neuroscience. 2007. PMID: 17258864 Free PMC article.
-
MPTP and DSP-4 susceptibility of substantia nigra and locus coeruleus catecholaminergic neurons in mice is independent of parkin activity.Neurobiol Dis. 2007 May;26(2):312-22. doi: 10.1016/j.nbd.2006.12.021. Epub 2007 Jan 25. Neurobiol Dis. 2007. PMID: 17336077 Free PMC article.
-
Evidence for a dissociation between MPTP toxicity and tyrosinase activity based on congenic mouse strain susceptibility.Exp Neurol. 2001 Mar;168(1):116-22. doi: 10.1006/exnr.2000.7588. Exp Neurol. 2001. PMID: 11170726
-
Behavioral phenotyping of the MPTP mouse model of Parkinson's disease.Behav Brain Res. 2001 Nov 1;125(1-2):109-25. doi: 10.1016/s0166-4328(01)00309-6. Behav Brain Res. 2001. PMID: 11682102 Review.
-
[Metabolic activation of azaheterocyclics induced dopaminergic toxicity: possible candidate neurotoxins underlying idiopathic Parkinson's disease].Nihon Hoigaku Zasshi. 1998 Oct;52(5):301-5. Nihon Hoigaku Zasshi. 1998. PMID: 10077975 Review. Japanese.
Cited by
-
Parkinson's disease mouse models in translational research.Mamm Genome. 2011 Aug;22(7-8):401-19. doi: 10.1007/s00335-011-9330-x. Epub 2011 May 11. Mamm Genome. 2011. PMID: 21559878 Free PMC article. Review.
-
Dominant inheritance of retinal ganglion cell resistance to optic nerve crush in mice.BMC Neurosci. 2007 Mar 5;8:19. doi: 10.1186/1471-2202-8-19. BMC Neurosci. 2007. PMID: 17338819 Free PMC article.
-
Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson's disease.Endocrine. 2006 Apr;29(2):275-87. doi: 10.1385/ENDO:29:2:275. Endocrine. 2006. PMID: 16785603
-
Admixing of MPTP-Resistant and Susceptible Mice Strains Augments Nigrostriatal Neuronal Correlates to Resist MPTP-Induced Neurodegeneration.Mol Neurobiol. 2017 Oct;54(8):6148-6162. doi: 10.1007/s12035-016-0158-y. Epub 2016 Oct 4. Mol Neurobiol. 2017. PMID: 27704331
-
Genetics of mouse behavioral and peripheral neural responses to sucrose.Mamm Genome. 2021 Apr;32(2):51-69. doi: 10.1007/s00335-021-09858-4. Epub 2021 Mar 13. Mamm Genome. 2021. PMID: 33713179
References
-
- Arvin M, Fedorkova L, Disshon KA, Dluzen DE, Leipheimer RE ( 2000) Estrogen modulates responses of striatal dopamine neurons to MPP+: evaluations using in vitro and in vivo techniques. Brain Res 872 : 160-171. - PubMed
-
- Belknap JK, Mitchell SR, O'Toole LA, Helms ML, Crabbe JC ( 1996) Type I and type II error rates for quantitative trait loci (QTL) mapping studies using recombinant inbred mouse strains. Behav Genet 26 : 149-160. - PubMed
-
- Chase K, Adler FR, Lark KG ( 1997) Epistat: a computer program for identifying and testing interactions between pairs of quantitative trait loci. Theor Appl Genet 94 : 724-730.
-
- Checkoway H, Farin FM, Costa MP, Kirchner SC, Costa LG ( 1998) Genetic polymorphisms in Parkinson's disease. Neurotoxicology 19 : 635-643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
