Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse
- PMID: 12967989
- PMCID: PMC6740689
- DOI: 10.1523/JNEUROSCI.23-23-08271.2003
Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse
Abstract
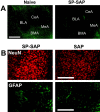
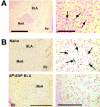
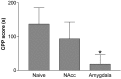
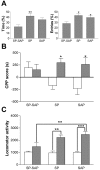
Mice lacking the neurokinin-1 (NK1) receptor, the preferred receptor for the neuropeptide substance P (SP), do not show many of the behaviors associated with morphine reward. To identify the areas of the brain that might contribute to this effect, we assessed the behavioral effects of ablation of neurons expressing the NK1 receptor in specific regions of the mouse brain using the neurotoxin substance P-saporin. In a preliminary investigation, bilateral ablation of these neurons from the amygdala, but not the nucleus accumbens and dorsomedial caudate putamen, brought about reductions in morphine reward behavior. Subsequently, the effect of ablation of these neurons in the amygdala on anxiety behavior was assessed using the elevated plus maze (EPM), before conditioned place preference (CPP), and locomotor responses to morphine were measured. Loss of NK1 receptor-expressing neurons in the amygdala caused an increase in anxiety-like behavior on the EPM. It also brought about a reduction in morphine CPP scores and the stimulant effect of acute morphine administration relative to saline controls, without affecting CPP to cocaine. NK1 receptor-expressing neurons in the mouse amygdala therefore modulate morphine reward behaviors. These observations mirror those observed in NK1 receptor knock-out (NK1-/-) mice and suggest that the amygdala is an important area for the effects of SP and the NK1 receptor in the motivational properties of opiates, as well as the control of behaviors related to anxiety.
Figures


Similar articles
-
NMDA Receptors on Dopaminoceptive Neurons Are Essential for Drug-Induced Conditioned Place Preference.eNeuro. 2016 Jun 9;3(3):ENEURO.0084-15.2016. doi: 10.1523/ENEURO.0084-15.2016. eCollection 2016 May-Jun. eNeuro. 2016. PMID: 27294197 Free PMC article.
-
Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors.Neuropharmacology. 2002 Dec;43(8):1258-68. doi: 10.1016/s0028-3908(02)00295-2. Neuropharmacology. 2002. PMID: 12527475
-
Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity.Brain Res. 1998 Apr 20;790(1-2):185-94. doi: 10.1016/s0006-8993(98)00062-6. Brain Res. 1998. PMID: 9593886
-
Neurobiology of substance P and the NK1 receptor.J Clin Psychiatry. 2002;63 Suppl 11:6-10. J Clin Psychiatry. 2002. PMID: 12562137 Review.
-
Mouse anxiety: the power of knockout.Pharmacogenomics J. 2001;1(3):187-92. doi: 10.1038/sj.tpj.6500016. Pharmacogenomics J. 2001. PMID: 11908755 Review.
Cited by
-
Impairment of skilled forelimb use after ablation of striatal interneurons expressing substance P receptors in rats: an analysis using a pasta matrix reaching task.Exp Brain Res. 2005 May;162(4):532-6. doi: 10.1007/s00221-004-2189-2. Epub 2005 Mar 8. Exp Brain Res. 2005. PMID: 15754181
-
Gene expression profiling in C57BL/6J and A/J mouse inbred strains reveals gene networks specific for brain regions independent of genetic background.BMC Genomics. 2010 Jan 11;11:20. doi: 10.1186/1471-2164-11-20. BMC Genomics. 2010. PMID: 20064228 Free PMC article.
-
Arsenic-Induced Activation of the Homeodomain-Interacting Protein Kinase 2 (HIPK2) to cAMP-Response Element Binding Protein (CREB) Axis.J Mol Biol. 2017 Jan 6;429(1):64-78. doi: 10.1016/j.jmb.2016.11.015. Epub 2016 Nov 21. J Mol Biol. 2017. PMID: 27884605 Free PMC article.
-
ProSAAS-derived peptides are regulated by cocaine and are required for sensitization to the locomotor effects of cocaine.J Neurochem. 2017 Nov;143(3):268-281. doi: 10.1111/jnc.14209. J Neurochem. 2017. PMID: 28881029 Free PMC article.
-
Neurokinin 1 and opioid receptors: relationships and interactions in nervous system.Transl Perioper Pain Med. 2016;1(3):11-21. Transl Perioper Pain Med. 2016. PMID: 28409174 Free PMC article.
References
-
- Alderson HL, Robbins TW, Everitt BJ ( 2000) The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology 153 : 111-119. - PubMed
-
- Bardo MT, Bevins RA ( 2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153 : 31-43. - PubMed
-
- Bechara A, van der Kooy D ( 1992) A single brain stem substrate mediates the motivational effects of both opiates and food in nondeprived rats but not in deprived rats. Behav Neurosci 106 : 351-363. - PubMed
-
- Ben-Ari Y, Zigmond RE, Moore KE ( 1975) Regional distribution of tyrosine hydroxylase, norepinephrine and dopamine within the amygdaloid complex of the rat. Brain Res 87 : 96-101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
