Activation of purinergic receptor subtypes modulates odor sensitivity
- PMID: 12967991
- PMCID: PMC2976511
- DOI: 10.1523/JNEUROSCI.23-23-08291.2003
Activation of purinergic receptor subtypes modulates odor sensitivity
Abstract
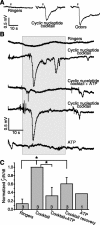
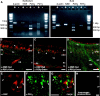
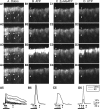
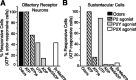
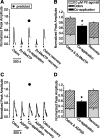
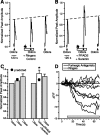
Purinergic nucleotides, including ATP and adenosine, are important neuromodulators of peripheral auditory and visual sensory systems (Thorne and Housley, 1996). ATP released by the olfactory epithelium (OE) after noxious stimuli provides a physiological source for a neuromodulatory substance independent of efferent innervation. Here we show that multiple subtypes of purinergic receptors are differentially expressed in olfactory receptor neurons and sustentacular support cells. Activation of purinergic receptors evoked inward currents and increases in intracellular calcium in cultured mouse olfactory receptor neurons. A mouse olfactory epithelial slice preparation and confocal imaging were used to measure changes in intracellular calcium in response to odors, purinergic receptor (P2R) agonists, or combined odor + P2R agonists. Pharmacological studies show that both P2Y and P2X receptor activation by exogenous and endogenous ATP significantly reduces odor responsiveness. Moreover, purinergic receptor antagonists increase the odor-evoked calcium transient, providing direct evidence that endogenous ATP modulates odor sensitivity via activation of multiple purinergic receptor subtypes in olfactory receptor neurons. Odor activation of G-protein-coupled receptors results in increased cAMP production, opening of cyclic nucleotide-gated channels, influx of Ca2+ and Na+, depolarization of the membrane, and activation of voltage- and Ca2+-gated ion channels. On-cell current-clamp recordings of olfactory receptor neurons from neonatal mouse slices revealed that ATP reduced cyclic nucleotide-induced electrical responses. These data also support the idea that ATP modulates odor sensitivity in mammalian olfactory neurons. Peripheral ATP-mediated odor suppression is a novel mechanism for reduced olfactory sensitivity during exposure to olfactotoxins and may be a novel neuroprotective mechanism.
Figures


Similar articles
-
Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels.Br J Pharmacol. 2000 Dec;131(8):1659-66. doi: 10.1038/sj.bjp.0703744. Br J Pharmacol. 2000. PMID: 11139444 Free PMC article.
-
Characterization of cultured dorsal root ganglion neuron P2X receptors.Eur J Neurosci. 1999 Jan;11(1):149-54. doi: 10.1046/j.1460-9568.1999.00426.x. Eur J Neurosci. 1999. PMID: 9987019
-
Nucleotide-induced Ca2+ signaling in sustentacular supporting cells of the olfactory epithelium.Glia. 2008 Nov 15;56(15):1614-24. doi: 10.1002/glia.20714. Glia. 2008. PMID: 18551628
-
Signaling by purinergic receptors and channels in the pituitary gland.Mol Cell Endocrinol. 2010 Jan 27;314(2):184-91. doi: 10.1016/j.mce.2009.05.008. Epub 2009 May 23. Mol Cell Endocrinol. 2010. PMID: 19467293 Free PMC article. Review.
-
Purinergic receptors in airway epithelia.Curr Opin Pharmacol. 2009 Jun;9(3):262-7. doi: 10.1016/j.coph.2009.02.004. Epub 2009 Mar 13. Curr Opin Pharmacol. 2009. PMID: 19285919 Free PMC article. Review.
Cited by
-
The Potential of Purinergic Signaling to Thwart Viruses Including SARS-CoV-2.Front Immunol. 2022 Jun 17;13:904419. doi: 10.3389/fimmu.2022.904419. eCollection 2022. Front Immunol. 2022. PMID: 35784277 Free PMC article. Review.
-
TMEM16A calcium-activated chloride currents in supporting cells of the mouse olfactory epithelium.J Gen Physiol. 2019 Jul 1;151(7):954-966. doi: 10.1085/jgp.201812310. Epub 2019 May 2. J Gen Physiol. 2019. PMID: 31048412 Free PMC article.
-
Constitutive and evoked release of ATP in adult mouse olfactory epithelium.Open Life Sci. 2024 Jan 16;19(1):20220811. doi: 10.1515/biol-2022-0811. eCollection 2024. Open Life Sci. 2024. PMID: 38250473 Free PMC article.
-
Follow Your Nose: A Key Clue to Understanding and Treating COVID-19.Front Endocrinol (Lausanne). 2021 Nov 18;12:747744. doi: 10.3389/fendo.2021.747744. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867791 Free PMC article.
-
Expression of P2X(2) and P2X (3) receptors in the rat carotid sinus, aortic arch, vena cava, and heart, as well as petrosal and nodose ganglia.Purinergic Signal. 2012 Mar;8(1):15-22. doi: 10.1007/s11302-011-9249-4. Epub 2011 Aug 5. Purinergic Signal. 2012. PMID: 21818574 Free PMC article.
References
-
- Bodin P, Burnstock G ( 2001) Purinergic signalling: ATP release. Neurochem Res 26 : 959-969. - PubMed
-
- Brake AJ, Wagenbach MJ, Julius D ( 1994) New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371 : 519-523. - PubMed
-
- Brandle U, Spielmanns P, Osteroth R, Sim J, Surprenant A, Buell G, Ruppersberg JP, Plinkert PK, Zenner HP, Glowatzki E ( 1997) Desensitization of the P2X(2) receptor controlled by alternative splicing. FEBS Lett 404 : 294-298. - PubMed
-
- Buck L, Axel R ( 1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65 : 175-187. - PubMed
-
- Burnstock G ( 1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36 : 1127-1139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
