Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats
- PMID: 12967992
- PMCID: PMC6740696
- DOI: 10.1523/JNEUROSCI.23-23-08302.2003
Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats
Abstract
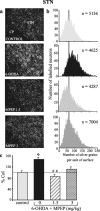
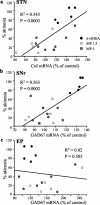
Glutamate overactivity within the basal ganglia has been shown to be central to the expression of motor symptoms in advanced stages of Parkinson's disease, and metabotropic glutamate receptors (mGluRs) represent promising targets for new therapeutic strategies in this pathology. Little is known, however, about the cellular and behavioral changes occurring in the early stages of the disease when dopamine depletion is moderate. Here, we report that rats with partial bilateral dopamine lesions exhibit akinetic deficits associated with dramatically increased neuronal metabolic activity in selective structures of the basal ganglia such as the subthalamic nucleus and the substantia nigra pars reticulata, but not in the entopeduncular nucleus. Furthermore, chronic treatment with the mGluR5 antagonist 2-methyl-6-(phenylethylnyl)-pyridine alleviated the akinesia and was associated with a normalization of the activity of these two overactive structures. These data stress the therapeutic potential of mGluR5 antagonists in the treatment of parkinsonian patients in the early stages of the disease.
Figures



References
-
- Amalric M, Berhow M, Polis I, Koob GF ( 1993) Selective effects of low-dose D2 dopamine receptor antagonism in a reaction-time task in rats. Neuropsychopharmacology 8 : 195-200. - PubMed
-
- Amalric M, Baunez C, Nieoullon A ( 1995a) Does the blockade of excitatory amino acid transmission in the basal ganglia simply reverse reaction time deficits induced by dopamine inactivation? Behav Pharmacol 6 : 508-519. - PubMed
-
- Amalric M, Moukhles H, Nieoullon A, Daszuta A ( 1995b) Complex deficits on reaction time performance following bilateral intrastriatal 6-OHDA infusion in the rat. Eur J Neurosci 7 : 972-980. - PubMed
-
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford NDP, Varney MA ( 2002) [3H] Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther 303 : 1044-1051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
