Evaluation of new therapies for inflammatory bowel disease
- PMID: 12968979
- PMCID: PMC1884373
- DOI: 10.1046/j.1365-2125.2003.01965.x
Evaluation of new therapies for inflammatory bowel disease
Erratum in
- Br J Clin Pharmacol. 2003 Nov;56(5):584
Abstract
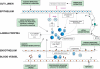
The pathophysiology of inflammatory bowel disease (IBD) is gradually being unravelled and new therapies are being developed to target the disturbed biological processes. This article outlines the clinical features of IBD, its current therapy and pathogenesis. The difficulties for clinical pharmacologists and gastroenterologists associated with designing, executing and interpreting clinical trials in IBD are then discussed. The final section reviews methods that can used to demonstrate the pharmacological actions of new treatments in patients with IBD. It is emphasized that proof of the therapeutic efficacy of a novel agent with a specific mechanism of action yields not only clinical benefit to patients with IBD, but also indicates the importance of the targeted biochemical pathway in the pathogenesis of the disease.
Figures
References
-
- Rampton DS, Shanahan F. Fast facts: Inflammatory bowel disease. Health Press; 2000.
-
- Moum B, Ekbom A, Vatn MH, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn's disease. Results of a large, prospective population- based study in southeastern Norway 1990–93. Scand J Gastroenterol. 1997;32:1005–1012. - PubMed
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Farrell RJ, Peppercorn MA. Ulcerative colitis. Lancet. 2002;359:331–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources