Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors
- PMID: 12970092
- PMCID: PMC1574038
- DOI: 10.1038/sj.bjp.0705442
Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors
Abstract
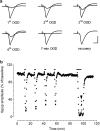
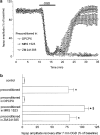
1. Ischemic preconditioning in the brain consists of reducing the sensitivity of neuronal tissue to further, more severe, ischemic insults. We recorded field epsps (fepsps) extracellularly from hippocampal slices to develop a model of in vitro ischemic preconditioning and to evaluate the role of A1, A2A and A3 adenosine receptors in this phenomenon. 2. The application of an ischemic insult, obtained by glucose and oxygen deprivation for 7 min, produced an irreversible depression of synaptic transmission. Ischemic preconditioning was induced by four ischemic insults (2 min each) separated by 13 min of normoxic conditions. After 30 min, an ischemic insult of 7 min was applied. This protocol substantially protected the tissue from the irreversible depression of synaptic activity. 3. The selective adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 100 nm), completely prevented the protective effect of preconditioning. The selective adenosine A2A receptor antagonist 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385, 100 nm) did not modify the magnitude of fepsp recovery compared to control slices. The selective A3 adenosine receptor antagonists, 3-propyl-6-ethyl-5[ethyl(thio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate (MRS 1523, 100 nm) significantly improved the recovery of fepsps after 7 min of ischemia. 4. Our results show that in vitro ischemic preconditioning allows CA1 hippocampal neurons to become resistant to prolonged exposure to ischemia. Adenosine, by stimulating A1 receptors, plays a crucial role in eliciting the cell mechanisms underlying preconditioning; A2A receptors are not involved in this phenomenon, whereas A3 receptor activation is harmful to ischemic preconditioning.
Figures



Comment in
-
Pre-conditioning protection in the brain.Br J Pharmacol. 2003 Sep;140(2):229-30. doi: 10.1038/sj.bjp.0705441. Epub 2003 Aug 11. Br J Pharmacol. 2003. PMID: 12970110 Free PMC article. No abstract available.
Similar articles
-
A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro.Br J Pharmacol. 2006 Mar;147(5):524-32. doi: 10.1038/sj.bjp.0706646. Br J Pharmacol. 2006. PMID: 16415905 Free PMC article.
-
Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices.Br J Pharmacol. 1999 Nov;128(5):1035-44. doi: 10.1038/sj.bjp.0702888. Br J Pharmacol. 1999. PMID: 10556941 Free PMC article.
-
The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices.Br J Pharmacol. 2009 Jul;157(5):818-30. doi: 10.1111/j.1476-5381.2009.00218.x. Epub 2009 May 5. Br J Pharmacol. 2009. PMID: 19422385 Free PMC article.
-
Purinergic signalling in brain ischemia.Neuropharmacology. 2016 May;104:105-30. doi: 10.1016/j.neuropharm.2015.11.007. Epub 2015 Nov 12. Neuropharmacology. 2016. PMID: 26581499 Review.
-
From A1 to A3 en passant through A(2A) receptors in the hippocampus: pharmacological implications.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):652-63. doi: 10.2174/187152712803581074. CNS Neurol Disord Drug Targets. 2012. PMID: 22963437 Review.
Cited by
-
Dexpramipexole improves bioenergetics and outcome in experimental stroke.Br J Pharmacol. 2018 Jan;175(2):272-283. doi: 10.1111/bph.13790. Epub 2017 May 12. Br J Pharmacol. 2018. PMID: 28320070 Free PMC article.
-
Nuclear Factor κB and Adenosine Receptors: Biochemical and Behavioral Profiling.Curr Neuropharmacol. 2011 Jun;9(2):342-9. doi: 10.2174/157015911795596559. Curr Neuropharmacol. 2011. PMID: 22131942 Free PMC article.
-
Microglial Adenosine Receptors: From Preconditioning to Modulating the M1/M2 Balance in Activated Cells.Cells. 2021 May 7;10(5):1124. doi: 10.3390/cells10051124. Cells. 2021. PMID: 34066933 Free PMC article. Review.
-
The role of ATP and adenosine in the brain under normoxic and ischemic conditions.Purinergic Signal. 2007 Sep;3(4):299-310. doi: 10.1007/s11302-007-9085-8. Epub 2007 Oct 11. Purinergic Signal. 2007. PMID: 18404443 Free PMC article.
-
Activation of Adenosine A1 Receptor in Ischemic Stroke: Neuroprotection by Tetrahydroxy Stilbene Glycoside as an Agonist.Antioxidants (Basel). 2021 Jul 12;10(7):1112. doi: 10.3390/antiox10071112. Antioxidants (Basel). 2021. PMID: 34356346 Free PMC article.
References
-
- ABBRACCHIO M.P., CATTABENI F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann. N.Y. Acad. Sci. 1999;890:79–92. - PubMed
-
- AKETA S., NAKASE H., KAMADA Y., HIRAMATSU K., SAKAKI T. Chemical preconditioning with 3-nitropropionic acid in gerbil hippocampal slices: therapeutic window and the participation of adenosine receptor. Exp. Neural. 2000;166:385–391. - PubMed
-
- ALICI K., WEBER-LUXENBURGER G., HEINEMANN U. Effects of glucose deprivation in area CA1 of hippocampal slices from adult and juvenile rats. Brain Res. Dev. Brain Res. 1998;107:71–80. - PubMed
-
- BLONDEAU N., PLAMONDON H., RICHELME C., HEURTEAUX C., LAZDUNSKI M. K(ATP) channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100:465–474. - PubMed
-
- BRAND A., VISSIENNON Z., ESCHKE D., NIEBER K. Adenosine A(1) and A(3) receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

