Potent analgesic effects of anticonvulsants on peripheral thermal nociception in rats
- PMID: 12970103
- PMCID: PMC1574030
- DOI: 10.1038/sj.bjp.0705434
Potent analgesic effects of anticonvulsants on peripheral thermal nociception in rats
Abstract
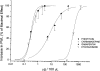
1. Anticonvulsant agents are commonly used to treat neuropathic pain conditions because of their effects on voltage- and ligand-gated channels in central pain pathways. However, their interaction with ion channels in peripheral pain pathways is poorly understood. Therefore, we studied the potential analgesic effects of commonly used anticonvulsant agents in peripheral nociception. 2. We injected anticonvulsants intradermally into peripheral receptive fields of sensory neurons in the hindpaws of adult rats, and studied pain perception using the model of acute thermal nociception. Commonly used anticonvulsants such as voltage-gated Na+ channel blockers, phenytoin and carbamazepine, and voltage-gated Ca2+ channel blockers, gabapentin and ethosuximide, induced dose-dependent analgesia in the injected paw, with ED50 values of 0.30, 0.32 and 8, 410 microg per 100 microl, respectively. 3. Thermal nociceptive responses were not affected in the contralateral, noninjected paws, indicating a lack of systemic effects with doses of anticonvulsants that elicited local analgesia. 4. Hill slope coefficients for the tested anticonvulsants indicate that the dose-response curve was less steep for gabapentin than for phenytoin, carbamazepine and ethosuximide. 5. Our data strongly suggest that cellular targets like voltage-gated Na+ and Ca2+ channels, similar to those that mediate the effects of anticonvulsant agents in the CNS, may exist in the peripheral nerve endings of rat sensory neurons. Thus, peripherally applied anticonvulsants that block voltage-gated Na+ and Ca2+ channels may be useful analgesics.
Figures


References
-
- BAKER M.D., WOOD J.N. Involvement of Na+ channels in pain pathways. TIPS. 2001;22:27–31. - PubMed
-
- CARLTON S.M., ZHOU S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain. 1998;76:201–207. - PubMed
-
- CARLTON S.M., ZHOU S., COGGESHALL R.E. Peripheral GABAA receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–722. - PubMed
-
- CODERRE T.J., KATZ J., VACCARINO A.L., MELZACK R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

