Molecular mechanisms of ligand-receptor interactions in transmembrane domain V of the alpha2A-adrenoceptor
- PMID: 12970108
- PMCID: PMC1574035
- DOI: 10.1038/sj.bjp.0705439
Molecular mechanisms of ligand-receptor interactions in transmembrane domain V of the alpha2A-adrenoceptor
Abstract
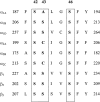
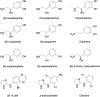
1. The structural determinants of catechol hydroxyl interactions with adrenergic receptors were examined using 12 alpha2-adrenergic agonists and a panel of mutated human alpha2A-adrenoceptors. The alpha2ASer201 mutant had a Cys --> Ser201 (position 5.43) amino-acid substitution, and alpha2ASer201Cys200 and alpha2ASer201Cys204 had Ser --> Cys200 (5.42) and Ser --> Cys204 (5.46) substitutions, respectively, in addition to the Cys --> Ser201 substitution. 2. Automated docking methods were used to predict the receptor interactions of the ligands. Radioligand-binding assays and functional [35S]GTPgammaS-binding assays were performed using transfected Chinese hamster ovary cells to experimentally corroborate the predicted binding modes. 3. The hydroxyl groups of phenethylamines were found to have different effects on ligand affinity towards the activated and resting forms of the wild-type alpha2A-adrenoceptor. Substitution of Ser200 or Ser204 with cysteine caused a deterioration in the capability of catecholamines to activate the alpha2A-adrenoceptor. The findings indicate that (i) Cys201 plays a significant role in the binding of catecholamine ligands and UK14,304 (for the latter, by a hydrophobic interaction), but Cys201 is not essential for receptor activation; (ii) Ser200 interacts with the meta-hydroxyl group of phenethylamine ligands, affecting both catecholamine binding and receptor activation; while (iii) substituting Ser204 with a cysteine interferes both with the binding of catecholamine ligands and with receptor activation, due to an interaction between Ser204 and the para-hydroxyl group of the catecholic ring.
Figures
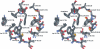


References
-
- AMBROSIO C., MOLINARI P., COTECCHIA S., COSTA T. Catechol-binding serines of β2-adrenergic receptors control the equilibrium between active and inactive receptor states. Mol. Pharmacol. 2000;57:198–210. - PubMed
-
- BALDWIN J.M., SCHERTLER G.F., UNGER V.M. An α-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J. Mol. Biol. 1997;272:144–164. - PubMed
-
- BALLESTEROS J.A., WEINSTEIN H.Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors Receptor Molecular Biology 1995San Diego: Academic Press, Inc; 366–427.ed. Sealfon, S.C. pp
-
- BERGMAN D.L., LAAKSONEN L., LAAKSONEN A. Visualizations of solvation structures in liquid mixtures. J. Mol. Graph. Model. 1997;15:301–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

