Structure of integrin alpha5beta1 in complex with fibronectin
- PMID: 12970173
- PMCID: PMC212714
- DOI: 10.1093/emboj/cdg445
Structure of integrin alpha5beta1 in complex with fibronectin
Abstract
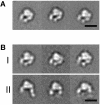
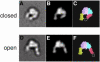
The membrane-distal headpiece of integrins has evolved to specifically bind large extracellular protein ligands, but the molecular architecture of the resulting complexes has not been determined. We used molecular electron microscopy to determine the three-dimensional structure of the ligand-binding headpiece of integrin alpha5beta1 complexed with fragments of its physiological ligand fibronectin. The density map for the unliganded alpha5beta1 headpiece shows a 'closed' conformation similar to that seen in the alphaVbeta3 crystal structure. By contrast, binding to fibronectin induces an 'open' conformation with a dramatic, approximately 80 degrees change in the angle of the hybrid domain of the beta subunit relative to its I-like domain. The fibronectin fragment binds to the interface between the beta-propeller and I-like domains in the integrin headpiece through the RGD-containing module 10, but direct contact of the synergy-region-containing module 9 to integrin is not evident. This finding is corroborated by kinetic analysis of real-time binding data, which shows that the synergy site greatly enhances k(on) but has little effect on the stability or k(off) of the complex.
Figures


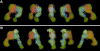
Similar articles
-
Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins.Biochem Soc Trans. 2004 Jun;32(Pt3):403-6. doi: 10.1042/BST0320403. Biochem Soc Trans. 2004. PMID: 15157147 Review.
-
Metal ion and ligand binding of integrin α5β1.Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17863-8. doi: 10.1073/pnas.1420645111. Epub 2014 Dec 4. Proc Natl Acad Sci U S A. 2014. PMID: 25475857 Free PMC article.
-
Relating conformation to function in integrin α5β1.Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3872-81. doi: 10.1073/pnas.1605074113. Epub 2016 Jun 17. Proc Natl Acad Sci U S A. 2016. PMID: 27317747 Free PMC article.
-
Crystal structure of α5β1 integrin ectodomain: atomic details of the fibronectin receptor.J Cell Biol. 2012 Apr 2;197(1):131-40. doi: 10.1083/jcb.201111077. Epub 2012 Mar 26. J Cell Biol. 2012. PMID: 22451694 Free PMC article.
-
Conformational regulation of integrin structure and function.Annu Rev Biophys Biomol Struct. 2002;31:485-516. doi: 10.1146/annurev.biophys.31.101101.140922. Epub 2001 Oct 25. Annu Rev Biophys Biomol Struct. 2002. PMID: 11988479 Review.
Cited by
-
Characterization of Endothelial Progenitor Cell Interactions with Human Tropoelastin.PLoS One. 2015 Jun 26;10(6):e0131101. doi: 10.1371/journal.pone.0131101. eCollection 2015. PLoS One. 2015. PMID: 26115013 Free PMC article.
-
A model for cyclic mechanical reinforcement.Sci Rep. 2016 Oct 27;6:35954. doi: 10.1038/srep35954. Sci Rep. 2016. PMID: 27786286 Free PMC article.
-
An RGD helper sequence in CagL of Helicobacter pylori assists in interactions with integrins and injection of CagA.Front Cell Infect Microbiol. 2012 May 23;2:70. doi: 10.3389/fcimb.2012.00070. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919661 Free PMC article.
-
Actin-binding protein filamin A is displayed on the surface of human neuroblastoma cells.Cancer Sci. 2006 Dec;97(12):1359-65. doi: 10.1111/j.1349-7006.2006.00327.x. Epub 2006 Sep 25. Cancer Sci. 2006. PMID: 16999820 Free PMC article.
-
Low-affinity integrin states have faster ligand-binding kinetics than the high-affinity state.Elife. 2021 Dec 2;10:e73359. doi: 10.7554/eLife.73359. Elife. 2021. PMID: 34854380 Free PMC article.
References
-
- Altroff H., van Der Walle,C.F., Asselin,J., Fairless,R., Campbell,I.D. and Mardon,H.J. (2001) The eighth FIII domain of human fibronectin promotes integrin α5β1 binding via stabilization of the ninth FIII domain. J. Biol. Chem., 276, 38885–38892. - PubMed
-
- Aota S., Nomizu,M. and Yamada,K.M. (1994) The short amino acid sequence Pro–His–Ser–Arg–Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem., 269, 24756–24761. - PubMed
-
- Baron M., Main,A.L., Driscoll,P.C., Mardon,H.J., Boyd,J. and Campbell,I.D. (1992) 1H NMR assignment and secondary structure of the cell adhesion type III module of fibronectin. Biochemistry, 31, 2068–2073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

