Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p
- PMID: 12970193
- PMCID: PMC212715
- DOI: 10.1093/emboj/cdg446
Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p
Abstract
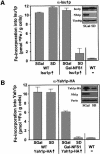
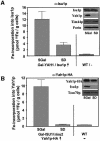
The mitochondrial proteins Isu1p and Isu2p play an essential role in the maturation of cellular iron-sulfur (Fe/S) proteins in eukaryotes. By radiolabelling of yeast cells with 55Fe we demonstrate that Isu1p binds an oxygen-resistant non-chelatable Fe/S cluster providing in vivo evidence for a scaffolding function of Isu1p during Fe/S cluster assembly. Depletion of the cysteine desulfurase Nfs1p, the ferredoxin Yah1p or the yeast frataxin homologue Yfh1p by regulated gene expression causes a strong decrease in the de novo synthesis of Fe/S clusters on Isu1p. In contrast, depletion of the Hsp70 chaperone Ssq1p, its co-chaperone Jac1p or the glutaredoxin Grx5p markedly increased the amount of Fe/S clusters bound to Isu1p, even though these mitochondrial proteins are crucial for maturation of Fe/S proteins. Hence Ssq1p/Jac1p and Grx5p are required in a step after Fe/S cluster synthesis on Isu1p, for instance in dissociation of preassembled Fe/S clusters from Isu1p and/or their insertion into apoproteins. We propose a model that dissects Fe/S cluster biogenesis into two major steps and assigns its central components to one of these two steps.
Figures






References
-
- Agar J.N., Krebs,C., Frazzon,J., Huynh,B.H., Dean,D.R. and Johnson,M.K. (2000a) IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry, 39, 7856–7862. - PubMed
-
- Agar J.N., Yuvaniyama,P., Jack,R.F., Cash,V.L., Smith,A.D., Dean,D.R. and Johnson,M.K. (2000b) Modular organization and identification of a mononuclear iron-binding site within the NifU protein. J. Biol. Inorg. Chem., 5, 167–177. - PubMed
-
- Agar J.N., Zheng,L., Cash,V.L., Dean,D.R. and Johnson,M.K. (2000c) Role of the IscU protein in iron–sulfur cluster biosynthesis: IscS-mediated assembly of a [Fe2S2] cluster in IscU. J. Am. Chem. Soc., 122, 2136–2137.
-
- Beinert H. and Kiley,P.J. (1999) Fe–S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol., 3, 152–157. - PubMed
-
- Beinert H., Holm,R.H. and Münck,E. (1997) Iron–sulfur clusters: Nature’s modular, multipurpose structures. Science, 277, 653–659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

