VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation
- PMID: 12970196
- PMCID: PMC212719
- DOI: 10.1093/emboj/cdg451
VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation
Abstract
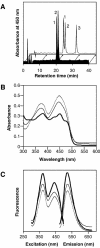
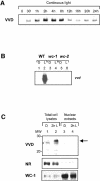
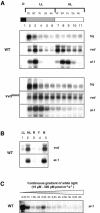
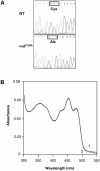
Blue light regulates many physiological and developmental processes in fungi. Most of the blue light responses in the ascomycete Neurospora crassa are dependent on the two blue light regulatory proteins White Collar (WC)-1 and -2. WC-1 has recently been shown to be the first fungal blue light photoreceptor. In the present study, we characterize the Neurospora protein VIVID. VIVID shows a partial sequence similarity with plant blue light photoreceptors. In addition, we found that VIVID non-covalently binds a flavin chromophore. Upon illumination with blue light, VIVID undergoes a photocycle indicative of the formation of a flavin-cysteinyl adduct. VVD is localized in the cytoplasm and is only present after light induction. A loss-of-function vvd mutant was insensitive to increases in light intensities. Furthermore, mutational analysis of the photoactive cysteine indicated that the formation of a flavin-cysteinyl adduct is essential for VIVID functions in vivo. Our results show that VIVID is a second fungal blue light photoreceptor which enables Neurospora to perceive and respond to daily changes in light intensity.
Figures



References
-
- Baima S., Macino,G. and Morelli,G. (1991) Photoregulation of the albino-3 gene in Neurospora crassa. J. Photochem. Photobiol. B, 11, 107–115. - PubMed
-
- Batschauer A. (1998) Photoreceptors of higher plants. Planta, 206, 479–492. - PubMed
-
- Baum J.A. and Giles,N.H. (1985) Genetic control of chromatin structure 5′ to the qa-x and qa-2 genes of Neurospora. J. Mol. Biol., 182, 79–89. - PubMed
-
- Briggs W.R. and Christie,J.M. (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci., 7, 204–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

