A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2)
- PMID: 12970197
- PMCID: PMC212721
- DOI: 10.1093/emboj/cdg453
A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2)
Abstract
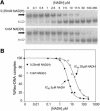
We describe the identification of Rex, a novel redox-sensing repressor that appears to be widespread among Gram-positive bacteria. In Streptomyces coelicolor Rex binds to operator (ROP) sites located upstream of several respiratory genes, including the cydABCD and rex-hemACD operons. The DNA-binding activity of Rex appears to be controlled by the redox poise of the NADH/NAD+ pool. Using electromobility shift and surface plasmon resonance assays we show that NADH, but not NAD+, inhibits the DNA-binding activity of Rex. However, NAD+ competes with NADH for Rex binding, allowing Rex to sense redox poise over a range of NAD(H) concentrations. Rex is predicted to include a pyridine nucleotide-binding domain (Rossmann fold), and residues that might play key structural and nucleotide binding roles are highly conserved. In support of this, the central glycine in the signature motif (GlyXGlyXXGly) is shown to be essential for redox sensing. Rex homologues exist in most Gram-positive bacteria, including human pathogens such as Staphylococcus aureus, Listeria monocytogenes and Streptococcus pneumoniae.
Figures







References
-
- Baker P.J., Britton,K.L., Rice,D.W., Rob,A. and Stillman,T.J. (1992) Structural consequences of sequence patterns in the fingerprint region of the nucleotide binding fold. Implications for nucleotide specificity. J. Mol. Biol., 228, 662–671. - PubMed
-
- Bentley S.D. et al. (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature, 417, 141–147. - PubMed
-
- Bierman M., Logan,R., O’Brien,K., Seno,E.T., Rao,R.N. and Schoner,B.E. (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene, 116, 43–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

