Enhanced virulence mediated by the murine coronavirus, mouse hepatitis virus strain JHM, is associated with a glycine at residue 310 of the spike glycoprotein
- PMID: 12970410
- PMCID: PMC228498
- DOI: 10.1128/jvi.77.19.10260-10269.2003
Enhanced virulence mediated by the murine coronavirus, mouse hepatitis virus strain JHM, is associated with a glycine at residue 310 of the spike glycoprotein
Abstract
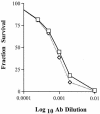
The coronavirus, mouse hepatitis virus strain JHM, causes acute and chronic neurological diseases in rodents. Here we demonstrate that two closely related virus variants, both of which cause acute encephalitis in susceptible strains of mice, cause markedly different diseases if mice are protected with a suboptimal amount of an anti-JHM neutralizing antibody. One strain, JHM.SD, caused acute encephalitis, while infection with JHM.IA resulted in no acute disease. Using recombinant virus technology, we found that the differences between the two viruses mapped to the spike (S) glycoprotein and that the two S proteins differed at four amino acids. By engineering viruses that differed by only one amino acid, we identified a serine-to-glycine change at position 310 of the S protein (S310G) that recapitulated the more neurovirulent phenotype. The increased neurovirulence mediated by the virus encoding glycine at position S310 was not associated with a different tropism within the central nervous system (CNS) but was associated with increased lateral spread in the CNS, leading to significantly higher brain viral titers. In vitro studies revealed that S310G was associated with decreased S1-S2 stability and with enhanced ability to mediate infection of cells lacking the primary receptor for JHM ("receptor-independent spread"). These enhanced fusogenic properties of viruses encoding a glycine at position 310 of the S protein may contribute to spread within the CNS, a tissue in which expression of conventional JHM receptors is low.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

