Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus
- PMID: 12970411
- PMCID: PMC228505
- DOI: 10.1128/jvi.77.19.10270-10279.2003
Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus
Abstract
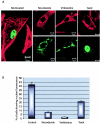
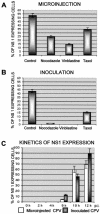
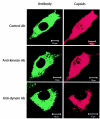
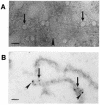
Canine parvovirus (CPV), a model virus for the study of parvoviral entry, enters host cells by receptor-mediated endocytosis, escapes from endosomal vesicles to the cytosol, and then replicates in the nucleus. We examined the role of the microtubule (MT)-mediated cytoplasmic trafficking of viral particles toward the nucleus. Immunofluorescence and immunoelectron microscopy showed that capsids were transported through the cytoplasm into the nucleus after cytoplasmic microinjection but that in the presence of MT-depolymerizing agents, viral capsids were unable to reach the nucleus. The nuclear accumulation of capsids was also reduced by microinjection of an anti-dynein antibody. Moreover, electron microscopy and light microscopy experiments demonstrated that viral capsids associate with tubulin and dynein in vitro. Coprecipitation studies indicated that viral capsids interact with dynein. When the cytoplasmic transport process was studied in living cells by microinjecting fluorescently labeled capsids into the cytoplasm of cells containing fluorescent tubulin, capsids were found in close contact with MTs. These results suggest that intact MTs and the motor protein dynein are required for the cytoplasmic transport of CPV capsids and contribute to the accumulation of the capsid in the nucleus.
Figures



Similar articles
-
Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus.J Cell Biol. 1997 Mar 10;136(5):1007-21. doi: 10.1083/jcb.136.5.1007. J Cell Biol. 1997. PMID: 9060466 Free PMC article.
-
Function of dynein and dynactin in herpes simplex virus capsid transport.Mol Biol Cell. 2002 Aug;13(8):2795-809. doi: 10.1091/mbc.01-07-0348. Mol Biol Cell. 2002. PMID: 12181347 Free PMC article.
-
Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport.J Virol. 2000 May;74(10):4853-9. doi: 10.1128/jvi.74.10.4853-4859.2000. J Virol. 2000. PMID: 10775624 Free PMC article.
-
HIV-1 capsid exploitation of the host microtubule cytoskeleton during early infection.Retrovirology. 2021 Jul 6;18(1):19. doi: 10.1186/s12977-021-00563-3. Retrovirology. 2021. PMID: 34229718 Free PMC article. Review.
-
Unchain my heart, baby let me go--the entry and intracellular transport of HIV.J Cell Biol. 2002 Nov 11;159(3):393-5. doi: 10.1083/jcb.200210024. Epub 2002 Nov 11. J Cell Biol. 2002. PMID: 12427864 Free PMC article. Review.
Cited by
-
Cytoplasmic Parvovirus Capsids Recruit Importin Beta for Nuclear Delivery.J Virol. 2020 Jan 31;94(4):e01532-19. doi: 10.1128/JVI.01532-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31748386 Free PMC article.
-
Highly dynamic microtubules improve the effectiveness of early stages of human influenza A/NWS/33 virus infection in LLC-MK2 cells.PLoS One. 2012;7(7):e41207. doi: 10.1371/journal.pone.0041207. Epub 2012 Jul 20. PLoS One. 2012. PMID: 22911759 Free PMC article.
-
Lipid-mediated introduction of hepatitis B virus capsids into nonsusceptible cells allows highly efficient replication and facilitates the study of early infection events.J Virol. 2006 Jun;80(11):5465-73. doi: 10.1128/JVI.02303-05. J Virol. 2006. PMID: 16699026 Free PMC article.
-
A common mechanism for cytoplasmic dynein-dependent microtubule binding shared among adeno-associated virus and adenovirus serotypes.J Virol. 2006 Aug;80(15):7781-5. doi: 10.1128/JVI.00481-06. J Virol. 2006. PMID: 16840360 Free PMC article.
-
Truncated forms of viral VP2 proteins fused to EGFP assemble into fluorescent parvovirus-like particles.J Nanobiotechnology. 2006 Dec 8;4:13. doi: 10.1186/1477-3155-4-13. J Nanobiotechnology. 2006. PMID: 17156442 Free PMC article.
References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Allan, V. 1996. Motor proteins: a dynamic duo. Curr. Biol. 6:630-633. - PubMed
-
- Alonso, C., J. Miskin, B. Hernaez, P. Fernandez-Zapatero, L. Soto, C. Canto, I. Rodriguez-Crespo, L. Dixon, and J. M. Escribano. 2001. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 75:9819-9827. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

