Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors
- PMID: 12970421
- PMCID: PMC228409
- DOI: 10.1128/jvi.77.19.10366-10375.2003
Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors
Abstract
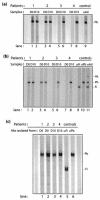
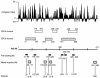
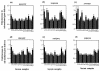
Sera from 17 patients with primary and secondary liver tumors who had been administered oncolytic adenovirus (Ad) mutant Addl1520 were analyzed for anti-Ad neutralization titers and antibodies to the Ad major capsid proteins hexon, penton base (Pb), and fiber. The antibodies recognized mainly conformational epitopes in hexon and both linear and conformational epitopes in Pb and fiber. Pb-specific antibodies were isolated from serum samples that had been obtained prior to and during the course of the treatment of four of these patients. We found that the Pb antibodies had a significant contribution toward anti-Ad neutralization, and this mainly occurred at the step of virus internalization. The Pb antigenic epitopes were determined by phage biopanning and were mapped to 10 discrete regions, which made up three major immunodominant domains within residues 51 to 120, 193 to 230, and 311 to 408, respectively. One of these domains (residues 311 to 408) overlapped the highly conserved, integrin-binding RGD (Arg-Gly-Asp) motif. The contribution of antibodies directed to RGD and other epitopes in Ad neutralization activity was determined indirectly by using a phage-mediated depletion assay. Our results suggested that circulating RGD antibodies were not prevalent and were poorly neutralizing and that other peptide motifs within residues 51 to 60, 216 to 226, and 311 to 408 in Pb sequence represented major target sites for neutralizing antibodies.
Figures
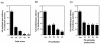
Similar articles
-
Immunoreactive domains and integrin-binding motifs in adenovirus penton base capsomer.Viral Immunol. 2000;13(3):353-71. doi: 10.1089/08828240050144671. Viral Immunol. 2000. PMID: 11016599
-
Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity.J Virol. 1998 Mar;72(3):2388-97. doi: 10.1128/JVI.72.3.2388-2397.1998. J Virol. 1998. PMID: 9499099 Free PMC article.
-
Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera.Virology. 2013 Mar 15;437(2):118-23. doi: 10.1016/j.virol.2012.12.014. Epub 2013 Jan 23. Virology. 2013. PMID: 23351390
-
The composed antigenic structure of the adenovirus hexon protein.Virologie. 1988 Oct-Dec;39(4):267-80. Virologie. 1988. PMID: 2464233 Review.
-
Antibody neutralization epitopes and integrin binding sites on nonenveloped viruses.Virology. 2001 Sep 30;288(2):189-91. doi: 10.1006/viro.2001.1095. Virology. 2001. PMID: 11601890 Review. No abstract available.
Cited by
-
Adenovirus Vectors: Excellent Tools for Vaccine Development.Immune Netw. 2021 Feb 15;21(1):e6. doi: 10.4110/in.2021.21.e6. eCollection 2021 Feb. Immune Netw. 2021. PMID: 33728099 Free PMC article. Review.
-
Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization.J Virol. 2010 Jan;84(1):630-8. doi: 10.1128/JVI.00866-09. J Virol. 2010. PMID: 19846512 Free PMC article.
-
Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach.Mol Pharm. 2011 Feb 7;8(1):3-11. doi: 10.1021/mp100214b. Epub 2010 Dec 1. Mol Pharm. 2011. PMID: 21047139 Free PMC article. Review.
-
Seroprevalence of neutralizing antibodies to adenovirus type 5 among children in India: implications for recombinant adenovirus-based vaccines.Clin Vaccine Immunol. 2007 Aug;14(8):1053-5. doi: 10.1128/CVI.00173-07. Epub 2007 Jun 27. Clin Vaccine Immunol. 2007. PMID: 17596429 Free PMC article.
-
Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions.J Virol. 2012 Jan;86(2):1267-72. doi: 10.1128/JVI.06165-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072746 Free PMC article.
References
-
- Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1B reading frames are required for the transformation of rodent cells by viral infection and DNA transfection. Virology 156:107-121. - PubMed
-
- Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373-376. - PubMed
-
- Boulanger, P., and F. Puvion. 1973. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur. J. Biochem. 39:37-42. - PubMed
-
- Chen, Y., D.-C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 11:1553-1567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

