Tissue-specific replicating capacity of a chimeric poliovirus that carries the internal ribosome entry site of hepatitis C virus in a new mouse model transgenic for the human poliovirus receptor
- PMID: 12970433
- PMCID: PMC228512
- DOI: 10.1128/jvi.77.19.10479-10487.2003
Tissue-specific replicating capacity of a chimeric poliovirus that carries the internal ribosome entry site of hepatitis C virus in a new mouse model transgenic for the human poliovirus receptor
Abstract
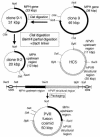
Nucleotides (nt) 108 to 742 of an infectious cDNA clone of poliovirus (PV) Mahoney strain, including the corresponding region of the internal ribosome entry site (IRES), was replaced by nt 28 to 710 of hepatitis C virus (HCV) cDNA corresponding to the whole HCV IRES. A chimeric PV (2A-369) was generated by transfecting mammalian cells with an RNA transcribed in vitro from the cDNA. To examine replicating capacity of virus 2A-369 in the brain and liver of a mouse model for poliomyelitis, a new mouse model (MPVRTg25-61) that is transgenic for human PV receptor (hPVR; CD155) was generated in order to obtain a higher expression level of hPVR in the liver than those of hPVRTg mouse lines generated by us so far. The transgene used was constructed by combining a putative regulatory region of the mouse PVR homolog and the whole structural region of the hPVR gene. Virus 2A-369 replicated well in the liver of MPVRTg25-61 but not in the brain, whereas control Mahoney virus replicated well both in the liver and in the brain. The data suggest that the HCV IRES works more efficiently in the liver than in the brain and that PV IRES works well both in the liver and in the brain. The results support the notion that tissue-specific activity of IRES may be reflected in tissue tropism of a virus whose specific translation initiation is driven by IRES, that is, an IRES-dependent virus tropism.
Figures





References
-
- Aoki, J., S. Koike, H. Asou, I. Ise, H. Suwa, T. Tanaka, M. Miyasaka, and A. Nomoto. 1997. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp. Cell Res. 235:374-384. - PubMed
-
- Eberle, F., P. Dubreuil, M. G. Mattei, E. Devilard, and M. Lopez. 1995. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 159:267-272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

