Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles
- PMID: 12970439
- PMCID: PMC228419
- DOI: 10.1128/jvi.77.19.10548-10556.2003
Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles
Abstract
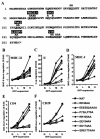
Recently, it has been demonstrated that the human immunodeficiency virus type 1 (HIV-1) Nef from laboratory strains down-modulates cell surface expression of mature major histocompatibility complex class II (MHC-II) molecules, while up-regulating surface expression of the invariant chain (Ii) associated with immature MHC-II (P. Stumptner-Cuvelette, S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch, Proc. Natl. Acad. Sci. USA 98:12144-12149, 2001). These Nef functions could contribute to impaired CD4(+)-T-helper-cell responses found in HIV-1-infected patients with progressive disease. However, it is currently unknown whether nef alleles derived from HIV-1-infected individuals or from other primate lentiviruses also modulate MHC-II and Ii. In the present study, we demonstrate that both activities are conserved among primary HIV-1 nef alleles, as well as among HIV-2 and simian immunodeficiency virus (SIV) nef alleles. Down-modulation of mature MHC-II required high levels of Nef expression. In contrast, surface expression of Ii was already strongly increased at low to medium levels of Nef expression. Notably, nef genes derived from two of four HIV-1-infected long-term nonprogressors did not up-regulate Ii, whereas nef alleles derived from 10 individuals with progressive disease were active in this assay. Unlike other in vitro Nef functions, the average activity of Nef in modulating MHC-II and Ii surface expression did not change significantly during the course of infection. Mutational analysis confirmed that MHC-II down- and Ii up-regulation are functionally separable from each other and from other Nef functions and identified acidic residues, located at the base of the flexible C-proximal loop of Nef, that are critical for increased Ii expression. Overall, our results suggest that the ability of Nef to interfere with MHC-II antigen presentation might play a role in AIDS pathogenesis.
Figures



Similar articles
-
Primary sooty mangabey simian immunodeficiency virus and human immunodeficiency virus type 2 nef alleles modulate cell surface expression of various human receptors and enhance viral infectivity and replication.J Virol. 2005 Aug;79(16):10547-60. doi: 10.1128/JVI.79.16.10547-10560.2005. J Virol. 2005. PMID: 16051847 Free PMC article.
-
Nef proteins from simian immunodeficiency virus-infected chimpanzees interact with p21-activated kinase 2 and modulate cell surface expression of various human receptors.J Virol. 2004 Jul;78(13):6864-74. doi: 10.1128/JVI.78.13.6864-6874.2004. J Virol. 2004. PMID: 15194762 Free PMC article.
-
Nef alleles from children with non-progressive HIV-1 infection modulate MHC-II expression more efficiently than those from rapid progressors.AIDS. 2007 May 31;21(9):1103-7. doi: 10.1097/QAD.0b013e32816aa37c. AIDS. 2007. PMID: 17502720
-
HIV/SIV escape from immune surveillance: focus on Nef.Curr HIV Res. 2004 Apr;2(2):141-51. doi: 10.2174/1570162043484924. Curr HIV Res. 2004. PMID: 15078178 Review.
-
Biology of the HIV Nef protein.Indian J Med Res. 2005 Apr;121(4):315-32. Indian J Med Res. 2005. PMID: 15817946 Review.
Cited by
-
One protein to rule them all: modulation of cell surface receptors and molecules by HIV Nef.Curr HIV Res. 2011 Oct;9(7):496-504. doi: 10.2174/157016211798842116. Curr HIV Res. 2011. PMID: 22103833 Free PMC article. Review.
-
HIV-1-Infected CD4+ T Cells Present MHC Class II-Restricted Epitope via Endogenous Processing.J Immunol. 2022 Sep 1;209(5):864-873. doi: 10.4049/jimmunol.2200145. Epub 2022 Aug 5. J Immunol. 2022. PMID: 36130133 Free PMC article.
-
Identification of a highly conserved valine-glycine-phenylalanine amino acid triplet required for HIV-1 Nef function.Retrovirology. 2012 Apr 27;9:34. doi: 10.1186/1742-4690-9-34. Retrovirology. 2012. PMID: 22537596 Free PMC article.
-
The efficiency of Vpx-mediated SAMHD1 antagonism does not correlate with the potency of viral control in HIV-2-infected individuals.Retrovirology. 2013 Mar 5;10:27. doi: 10.1186/1742-4690-10-27. Retrovirology. 2013. PMID: 23497283 Free PMC article.
-
Lentiviral Nef suppresses iron uptake in a strain specific manner through inhibition of Transferrin endocytosis.Retrovirology. 2014 Jan 2;11:1. doi: 10.1186/1742-4690-11-1. Retrovirology. 2014. PMID: 24383984 Free PMC article.
References
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Alexander, L., E. Weiskopf, T. C. Greenough, N. C. Gaddis, M. R. Auerbach, M. H. Malim, S. J. O'Brien, B. D. Walker, J. L. Sullivan, and R. C. Desrosiers. 2000. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 74:4361-4376. - PMC - PubMed
-
- Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717-2727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

