Differential regulation of glucose-6-phosphate dehydrogenase isoenzyme activities in potato
- PMID: 12970474
- PMCID: PMC196576
- DOI: 10.1104/pp.103.025676
Differential regulation of glucose-6-phosphate dehydrogenase isoenzyme activities in potato
Abstract
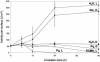
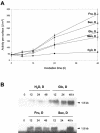
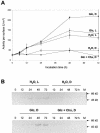
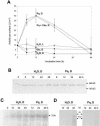
In plants, Glc-6-phosphate dehydrogenase (G6PDH) isoenzymes are present in the cytosol and in plastids. The plastidic enzymes (P1 and P2) are subject to redox regulation, but mechanisms that adjust cytosolic G6PDH activity are largely unknown. We adopted a leaf disc system for monitoring the effects of various conditions on G6PD isoform expression and enzyme activities in potato (Solanum tuberosum). Cytosolic G6PDH activity remained constant during water incubation in the dark. In continuous light or in the presence of metabolizable sugars in the dark, cytosolic G6PDH activity increased 6-fold within 24 h. Cycloheximide incubation demonstrated that enhanced cytosolic G6PDH activity depends on de novo protein synthesis. Osmotic change, phosphate sequestration, or oxidative stress did not affect cytosolic G6PDH activity. Furthermore, enzyme activity and protein contents closely followed the corresponding mRNA levels. Together with the fact that multiple SURE elements are present in the promoter region of the gene, these results suggest that cytosolic G6PDH activity is regulated by sugar availability at the transcriptional level. Plastidic G6PDH activity stayed constant during water incubation in the light and dropped to minimal levels within 6 h in the dark. Conversely, plastidic G6PDH activity of leaf discs incubated on Paraquat rose to 10-fold higher levels, which was not prevented by cycloheximide. Similar increases were found with nitrite, nitrate, or sulfate. No major changes in protein or mRNA contents of the plastidic P1 and P2 isoforms were registered. K(m) (Glc-6-phosphate) values of plastidic G6PDH activity differed between samples incubated on water or Paraquat, suggesting posttranslational modification of the plastidic enzyme(s). Immunoprecipitation of (32)P-labeled samples with P1 isoform-specific antibodies showed that the chloroplast enzyme is subject to protein phosphorylation. Obviously, in extended dark periods, G6PDH activity in the stroma is restricted but can be stimulated in response to high demands for NADPH.
Figures



References
-
- Anderson DJ, Blobel G (1983) Immunoprecipitation of proteins from cell-free translations. Methods Enzymol 96: 111-120 - PubMed
-
- Ashton AR, Burnell JN, Hatch MD (1984) Regulation of C4 photosynthesis: inactivation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and activation by phosphorolysis. Arch Biochem Biophys 230: 492-503 - PubMed
-
- Aubert S, Gout E, Bligny R, Douce R (1994) Multiple effects of glycerol on plant cell metabolism. J Biol Chem 269: 21420-21427 - PubMed
-
- Baginsky S, Tiller K, Link G (1997) Transcription factor phosphorylation by a protein kinase associated with chloroplast RNA polymerase from mustard (Sinapis alba). Plant Mol Biol 34: 181-189 - PubMed
-
- Batz O, Logemann E, Reinold S, Hahlbrock K (1998) Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. Biol Chem 379: 1127-1135 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

