The polycotyledon mutant of tomato shows enhanced polar auxin transport
- PMID: 12970479
- PMCID: PMC196586
- DOI: 10.1104/pp.103.025478
The polycotyledon mutant of tomato shows enhanced polar auxin transport
Abstract
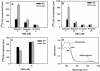
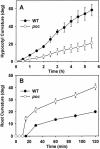
The polycotyledon mutant of tomato (Lycopersicon esculentum L. cv Ailsa Craig) showed altered development during embryogenesis and during vegetative and reproductive phases. The phenotype was pleiotropic and included the formation of extra cotyledons, changes in leaf shape, increased number of flowers (indeterminacy) with abnormal floral organs, the formation of epiphyllous structures, and altered gravitropism. The earliest defects were observed at the transition from the globular to the heart stage of embryogenesis with the formation of multiple cotyledons. Epidermal cells in the mutant embryo were smaller and less expanded compared with wild type. Examination of polar auxin transport (PAT) showed a striking enhancement in the case of the mutant. Increase in PAT did not appear to be caused by a decrease in flavonoids because the mutant had normal flavonoid levels. Application of 2,3,5-triiodobenzoic acid, an inhibitor of polar transport of auxin, rescued postgermination phenotypes of young seedlings. Our analysis reveals a level of control that negatively regulates PAT in tomato and its contribution to plant development and organogenesis.
Figures





References
-
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057-4067 - PubMed
-
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease like regulator of root gravitropism. Science 273: 948-950 - PubMed
-
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505-520
-
- Berleth T, Chatfield S (2002) Embryogenesis: pattern formation from a single cell. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, http://www.aspb.org/downlaods/arabidopsis/berleth.pdf - PMC - PubMed
-
- Berleth T, Jurgens G (1993) The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development 118: 575-587
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

