Intravenous ibandronate injections given every three months: a new treatment option to prevent bone loss in postmenopausal women
- PMID: 12972476
- PMCID: PMC1754320
- DOI: 10.1136/ard.62.10.969
Intravenous ibandronate injections given every three months: a new treatment option to prevent bone loss in postmenopausal women
Abstract
Objective: To investigate the efficacy, safety, and dose response of three doses of ibandronate, given intermittently by intravenous (IV) injection every three months, in preventing postmenopausal osteoporosis.
Patients and methods: 629 postmenopausal women, categorised according to time since menopause and baseline lumbar spine (L1-4) bone mineral density (BMD), were enrolled into a multicentre, double blind, placebo controlled trial. They were randomly allocated to receive IV ibandronate 0.5 mg, 1 mg or 2 mg, or placebo every three months. All women received daily calcium supplementation.
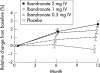
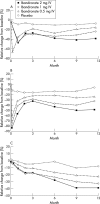
Results: One year's treatment with intermittent IV ibandronate injections produced a dose dependent gain in mean (SD) lumbar spine BMD from baseline of 2.5 (2.5)%, 1.8 (2.6)%, and 1.0 (2.8)% in the groups receiving 2 mg, 1 mg, and 0.5 mg ibandronate, respectively, compared with a loss of BMD of 0.4 (2.4)% in the women in the placebo group; p=0.0001 for each ibandronate dose v placebo. Highest BMD gains occurred in women with osteopenia receiving 2 mg ibandronate. Similarly, at the hip, all three doses of ibandronate produced significantly better gains in BMD than placebo (p<0.05), with the greatest gains in the women with osteopenia receiving the 2 mg dose. Ibandronate concomitantly and dose dependently suppressed markers of bone turnover in comparison with placebo, and injections were well tolerated.
Conclusion: IV ibandronate injections, given every three months, may be an effective alternative to oral bisphosphonates and hormonal therapy in the prevention of bone loss in postmenopausal women.
Figures
Comment in
-
Ibandronate and prevention of postmenopausal osteoporosis.Ann Rheum Dis. 2004 May;63(5):608-9; author reply 609-10. Ann Rheum Dis. 2004. PMID: 15082505 Free PMC article. No abstract available.
Similar articles
-
Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis.J Bone Miner Res. 2004 Jan;19(1):11-8. doi: 10.1359/JBMR.0301202. J Bone Miner Res. 2004. PMID: 14753731 Clinical Trial.
-
Oral weekly ibandronate prevents bone loss in postmenopausal women.J Intern Med. 2003 Aug;254(2):159-67. doi: 10.1046/j.1365-2796.2003.01174.x. J Intern Med. 2003. PMID: 12859697 Clinical Trial.
-
The effect on bone mass and bone markers of different doses of ibandronate: a new bisphosphonate for prevention and treatment of postmenopausal osteoporosis: a 1-year, randomized, double-blind, placebo-controlled dose-finding study.Bone. 1996 Nov;19(5):527-33. doi: 10.1016/s8756-3282(96)00229-3. Bone. 1996. PMID: 8922653 Clinical Trial.
-
Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies.Clin Ther. 2003 Jan;25(1):19-34. doi: 10.1016/s0149-2918(03)90005-1. Clin Ther. 2003. PMID: 12637110 Review.
-
Oral and intravenous ibandronate in the management of postmenopausal osteoporosis: a comprehensive review.Curr Pharm Des. 2005;11(28):3711-28. doi: 10.2174/138161205774580750. Curr Pharm Des. 2005. PMID: 16305506 Review.
Cited by
-
Validation of the Surrogate Threshold Effect for Change in Bone Mineral Density as a Surrogate Endpoint for Fracture Outcomes: The FNIH-ASBMR SABRE Project.J Bone Miner Res. 2022 Jan;37(1):29-35. doi: 10.1002/jbmr.4433. Epub 2021 Sep 24. J Bone Miner Res. 2022. PMID: 34490915 Free PMC article.
-
Effective and rapid treatment of painful localized transient osteoporosis (bone marrow edema) with intravenous ibandronate.Osteoporos Int. 2005 Dec;16(12):2063-8. doi: 10.1007/s00198-005-2001-6. Epub 2005 Oct 14. Osteoporos Int. 2005. PMID: 16228105
-
Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing.Osteoporos Int. 2004 Jun;15(6):423-33. doi: 10.1007/s00198-004-1612-7. Epub 2004 Mar 26. Osteoporos Int. 2004. PMID: 15205712 Review.
-
Intravenous ibandronate in the treatment of osteoporosis: profile report.Drugs Aging. 2006;23(12):997-1001. doi: 10.2165/00002512-200623120-00006. Drugs Aging. 2006. PMID: 17154663 No abstract available.
-
Intravenous ibandronate: in the treatment of osteoporosis.Drugs. 2006;66(12):1593-601; discussion 1602-3. doi: 10.2165/00003495-200666120-00005. Drugs. 2006. PMID: 16956306 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources