Rrp47p is an exosome-associated protein required for the 3' processing of stable RNAs
- PMID: 12972615
- PMCID: PMC193929
- DOI: 10.1128/MCB.23.19.6982-6992.2003
Rrp47p is an exosome-associated protein required for the 3' processing of stable RNAs
Abstract
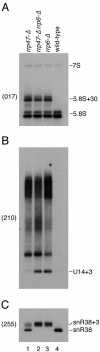
Related exosome complexes of 3'-->5' exonucleases are present in the nucleus and the cytoplasm. Purification of exosome complexes from whole-cell lysates identified a Mg(2+)-labile factor present in substoichiometric amounts. This protein was identified as the nuclear protein Yhr081p, the homologue of human C1D, which we have designated Rrp47p (for rRNA processing). Immunoprecipitation of epitope-tagged Rrp47p confirmed its interaction with the exosome and revealed its association with Rrp6p, a 3'-->5' exonuclease specific to the nuclear exosome fraction. Northern analyses demonstrated that Rrp47p is required for the exosome-dependent processing of rRNA and small nucleolar RNA (snoRNA) precursors. Rrp47p also participates in the 3' processing of U4 and U5 small nuclear RNAs (snRNAs). The defects in the processing of stable RNAs seen in rrp47-Delta strains closely resemble those of strains lacking Rrp6p. In contrast, Rrp47p is not required for the Rrp6p-dependent degradation of 3'-extended nuclear pre-mRNAs or the cytoplasmic 3'-->5' mRNA decay pathway. We propose that Rrp47p functions as a substrate-specific nuclear cofactor for exosome activity in the processing of stable RNAs.
Figures




References
-
- Bachellerie, J. P., J. Cavaille, and A. Huttenhofer. 2002. The expanding snoRNA world. Biochimie 84:775-790. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
