Proton transport in maize tonoplasts supported by fructose-1,6-bisphosphate cleavage. Pyrophosphate-dependent phosphofructokinase as a pyrophosphate-regenerating system
- PMID: 12972651
- PMCID: PMC219061
- DOI: 10.1104/pp.103.026633
Proton transport in maize tonoplasts supported by fructose-1,6-bisphosphate cleavage. Pyrophosphate-dependent phosphofructokinase as a pyrophosphate-regenerating system
Abstract
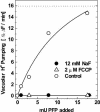
The energy derived from pyrophosphate (PPi) hydrolysis is used to pump protons across the tonoplast membrane, thus forming a proton gradient. In a plant's cytosol, the concentration of PPi varies between 10 and 800 microm, and the PPi concentration needed for one-half maximal activity of the maize (Zea mays) root tonoplast H+-pyrophosphatase is 30 microm. In this report, we show that the H+-pyrophosphatase of maize root vacuoles is able to hydrolyze PPi (Reaction 2) formed by Reaction 1, which is catalyzed by PPi-dependent phosphofructokinase (PFP): Fructose-1,6-bisphosphate (F1,6BP) + Pi <--> PPi +Fructose-6-phosphate (F6 P) (reaction 1) PPi --> 2 Pi (reaction 2) H+cyt --> H+vac (reaction 3) F1,6BP + H+cyt <--> H+vac + F6P + Pi (reaction 4) During the steady state, one-half of the inorganic phosphate released (Reaction 4) is ultimately derived from F1,6BP, whereas PFP continuously regenerates the pyrophosphate (PPi) hydrolyzed. A proton gradient (DeltapH) can be built up in tonoplast vesicles using PFP as a PPi-regenerating system. The Delta pH formed by the H+-pyrophosphatase can be dissipated by addition of 20 mm F6P, which drives Reaction 1 to the left and decreases the PPi available for the H+-pyrophosphatase. The maximal Delta pH attained by the pyrophosphatase coupled to the PFP reaction can be maintained by PFP activities far below those found in higher plants tissues.
Figures




Similar articles
-
Kinetic mechanism of pyrophosphate-dependent phosphofructokinase from Giardia lamblia.Mol Biochem Parasitol. 1995 Jul;73(1-2):43-51. doi: 10.1016/0166-6851(95)00087-h. Mol Biochem Parasitol. 1995. PMID: 8577346
-
Purification and characterization of pyrophosphate- and ATP-dependent phosphofructokinases from banana fruit.Planta. 2003 May;217(1):113-21. doi: 10.1007/s00425-002-0962-7. Epub 2003 Jan 14. Planta. 2003. PMID: 12721855
-
Chilling induces a decrease in pyrophosphate-dependent H+-accumulation associated with a DeltapH(vac)-stat in mung bean, a chill-sensitive plant.Plant Cell Environ. 2008 Mar;31(3):288-300. doi: 10.1111/j.1365-3040.2007.01762.x. Epub 2007 Nov 22. Plant Cell Environ. 2008. PMID: 18034771
-
Role of water in processes of energy transduction: Ca2+-transport ATPase and inorganic pyrophosphatase.Biochem Soc Symp. 1985;50:97-125. Biochem Soc Symp. 1985. PMID: 2428374 Review.
-
The Mechanism of Energy Coupling in H+/Na+-Pumping Membrane Pyrophosphatase-Possibilities and Probabilities.Int J Mol Sci. 2022 Aug 22;23(16):9504. doi: 10.3390/ijms23169504. Int J Mol Sci. 2022. PMID: 36012762 Free PMC article. Review.
Cited by
-
Inorganic phosphate uptake in intact vacuoles isolated from suspension-cultured cells of Catharanthus roseus (L.) G. Don under varying Pi status.Planta. 2007 Feb;225(3):711-8. doi: 10.1007/s00425-006-0379-9. Epub 2006 Sep 6. Planta. 2007. PMID: 16955272
-
Low levels of pyrophosphate in transgenic potato plants expressing E. coli pyrophosphatase lead to decreased vitality under oxygen deficiency.Ann Bot. 2005 Sep;96(4):717-26. doi: 10.1093/aob/mci223. Epub 2005 Jul 18. Ann Bot. 2005. PMID: 16027130 Free PMC article.
References
-
- Baykov AA, Cooperman BS, Goldman A, Lahti R (1999) Cytoplasmic inorganic pyrophosphatase. Prog Mol Subcell Biol 23: 127–150 - PubMed
-
- Black CC, Mustardy L, Suna SS, Kormanik PP, Xu D-P, Paz N (1987) Regulation and roles for alternative pathways of hexose metabolism in plants. Physiol Plant 69: 387–394
-
- Clayton H, Ranson J, ap Rees T (1993) Pyrophosphate-fructose-6-phosphate 1-phosphotransferase and fructose 2,6-bisphosphate in the bundle sheath of maize leaves. Arch Biochem Biophys 301: 151–157 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

