Delivery of antigen in allogeneic cells preferentially generates CD(4+) Th1 cells
- PMID: 12974749
- PMCID: PMC1808824
- DOI: 10.1046/j.1365-2249.2003.02254.x
Delivery of antigen in allogeneic cells preferentially generates CD(4+) Th1 cells
Abstract
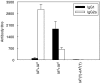
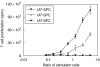
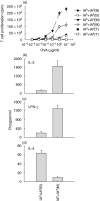
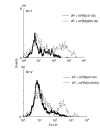
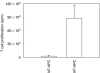
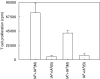
We have examined the possibility of evoking antigen-specific T cell immune response by using allogeneic cells as a source of adjuvant and also as a vehicle to deliver antigen. The mice were immunized with different preparations of antigen-pulsed allogeneic and syngeneic splenocytes. It was observed during the study that the animals immunized with antigen-pulsed mitomycin C treated allogeneic cells elicited antigen specific CD(4+) Th1 cell response. Predominant release of IL-2, interferon (IFN)-gamma and IgG2a-isotype also occurred. In contrast, mice immunized with antigen-pulsed syngeneic cells chiefly enhanced the production of interleukin (IL)-4 and IgG1-isotype. Further, allogeneic macrophages induced better T cell response than B cells or splenocytes and prominently induced the expression of B7-1 and B7-2. Immunization with antigen-pulsed macrophages provided better recall responses compared to B cells. This was manifested by the high LFA-1alpha and low CD45RB expression on T cells. Because it is already known that mitomycin C-treated cells undergo apoptosis and dendritic cells engulf apoptotic cells, we therefore propose that generation of T cell response using antigen-pulsed allogeneic cells may be due to the engulfment of these cells by dendritic cells, which may then process and present antigen entrapped in allogeneic cells to activate naive CD(4+) T cells and differentiate them to Th1 cells. This study therefore provides a rational basis for manipulating antigen-specific responses by immunizing with antigen-pulsed allogeneic cells.
Figures


Similar articles
-
Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28.Int Immunol. 1998 May;10(5):651-62. doi: 10.1093/intimm/10.5.651. Int Immunol. 1998. PMID: 9645613
-
Selective development of T helper (Th)2 cells induced by continuous administration of low dose soluble proteins to normal and beta(2)-microglobulin-deficient BALB/c mice.J Exp Med. 1996 Feb 1;183(2):485-97. doi: 10.1084/jem.183.2.485. J Exp Med. 1996. PMID: 8627161 Free PMC article.
-
Lack of Th1 or Th2 polarization of CD4+ T cell response induced by particulate antigen targeted to phagocytic cells.Int Immunol. 1997 Jan;9(1):91-103. doi: 10.1093/intimm/9.1.91. Int Immunol. 1997. PMID: 9043951
-
Regulatory role of pro-Th1 and pro-Th2 cytokines in modulating the activity of Th1 and Th2 cells when B cell and macrophages are used as antigen presenting cells.BMC Immunol. 2006 Aug 7;7:17. doi: 10.1186/1471-2172-7-17. BMC Immunol. 2006. PMID: 16889674 Free PMC article.
-
Role of Th1 and Th2 cells in anterior chamber-associated immune deviation.Immunology. 1996 Sep;89(1):34-40. doi: 10.1046/j.1365-2567.1996.d01-714.x. Immunology. 1996. PMID: 8911137 Free PMC article.
Cited by
-
Co-administration of IL-1+IL-6+TNF-α with Mycobacterium tuberculosis infected macrophages vaccine induces better protective T cell memory than BCG.PLoS One. 2011 Jan 19;6(1):e16097. doi: 10.1371/journal.pone.0016097. PLoS One. 2011. PMID: 21283805 Free PMC article.
-
Unexpected impairment of TNF-α-induced maturation of human dendritic cells in vitro by IL-4.J Transl Med. 2016 Apr 14;14:93. doi: 10.1186/s12967-016-0848-2. J Transl Med. 2016. PMID: 27080531 Free PMC article.
References
-
- O'Garra A. Cytokines induce the development of functionally heterogenous T helper subsets. Immunity. 1998;8:275–83. - PubMed
-
- Agrewala JN, Suvas S, Verma RK, Mishra GC. Differential effect of anti-B7-1 and anti-M150 antibodies in restricting the delivery of co-stimulatory signals from B cells and macrophages. J Immunol. 1998;160:1067–77. - PubMed
-
- Agrewala JN, Wilkinson RJ. Influence of HLA-DR on the phenotype of CD4+ T lymphocytes specific for an epitope of the 16-kD a-crystalline antigen of Mycobacterium tuberculosis. Eur J Immunol. 1999;29:1753–61. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

