Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis
- PMID: 12974765
- PMCID: PMC1808826
- DOI: 10.1046/j.1365-2249.2003.02268.x
Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis
Abstract
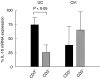
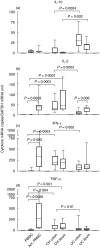
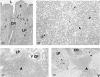
Ulcerative colitis (UC), a chronic inflammatory bowel disease, exhibits pronounced increase of T lymphocytes in the inflamed mucosa. To understand the role of intestinal T lymphocytes in the pathogenesis of UC their cytokine production in the mucosa was analysed. Intestinal T lymphocytes of UC, Crohn's disease and control patients were analysed for cytokine mRNA levels by real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) directly after isolation without in vitro stimulation. Frequencies of cytokine positive cells were determined in UC and control colon by immunomorphometry. T lymphocytes in normal colon expressed interleukin (IL)-2, interferon (IFN)-gamma, tumour necrosis factor (TNF)-alpha and transforming growth factor (TGF)-beta1, but not IL-4, IL-5 or IL-10. In UC, a highly significant increase in IL-10 mRNA levels in T lymphocytes and an increased frequency of IL-10 positive cells was seen in colon. IL-10 mRNA levels were also elevated in T lymphocytes of the non-inflamed ileum and correlated with disease activity at both locations. CD4+ T lymphocytes were the major source of IL-10 mRNA. IL-2, IFN-gamma and TNF-alpha mRNA levels were decreased in colonic T lymphocytes, and virtually no IL-2, IFN-gamma, TNF-alpha or TGF-beta positive cells were detected in basal lymphoid aggregates. However, scattered IL-10 positive cells were found here. Lamina propria outside the aggregates contained IL-10-, IFN-gamma, TNF-alpha and TGF-beta but not IL-2 positive cells. T cells of UC patients did not express IL-4 or IL-5. Taken, together the data suggest a generalized activation of IL-10 producing CD4+ T cells along the intestine of UC patients. The local environment seems to determine the biological consequences of elevated IL-10.
Figures



References
-
- Fiocchi C. Inflammatory bowel disease. Etiol Pathogen Gastroenterol. 1998;115:182–505. - PubMed
-
- Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98:1264–71. - PubMed
-
- Kozarek RA. Review article: immunosuppressive therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7:117–23. - PubMed
-
- Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267–78. - PubMed
-
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 Scid mice. Int Immunol. 1993;11:1461–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

