Centrosome positioning in interphase cells
- PMID: 12975343
- PMCID: PMC2172857
- DOI: 10.1083/jcb.200305082
Centrosome positioning in interphase cells
Abstract
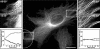
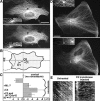
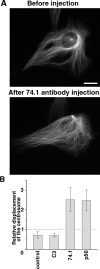
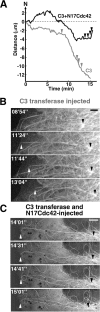
The position of the centrosome is actively maintained at the cell center, but the mechanisms of the centering force remain largely unknown. It is known that centrosome positioning requires a radial array of cytoplasmic microtubules (MTs) that can exert pushing or pulling forces involving MT dynamics and the activity of cortical MT motors. It has also been suggested that actomyosin can play a direct or indirect role in this process. To examine the centering mechanisms, we introduced an imbalance of forces acting on the centrosome by local application of an inhibitor of MT assembly (nocodazole), and studied the resulting centrosome displacement. Using this approach in combination with microinjection of function-blocking probes, we found that a MT-dependent dynein pulling force plays a key role in the positioning of the centrosome at the cell center, and that other forces applied to the centrosomal MTs, including actomyosin contractility, can contribute to this process.
Figures
References
-
- Cramer, L.P. 1997. Molecular mechanism of actin-dependent retrograde flow in lamellipodia of motile cells. Front. Biosci. 2:d260–d270. - PubMed
-
- Dogterom, M., and B. Yurke. 1997. Measurement of force-velocity relation for growing microtubules. Science. 278:856–860. - PubMed
-
- Dujardin, D.L., and R.B. Vallee. 2002. Dynein at the cortex. Curr. Opin. Cell Biol. 14:44–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

