Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers
- PMID: 12975355
- PMCID: PMC2172849
- DOI: 10.1083/jcb.200305078
Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers
Abstract
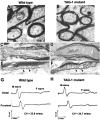
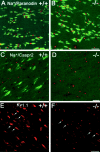
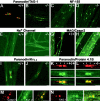
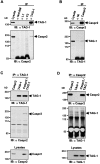
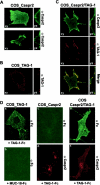
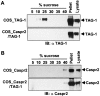
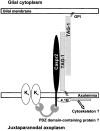
Myelination results in a highly segregated distribution of axonal membrane proteins at nodes of Ranvier. Here, we show the role in this process of TAG-1, a glycosyl-phosphatidyl-inositol-anchored cell adhesion molecule. In the absence of TAG-1, axonal Caspr2 did not accumulate at juxtaparanodes, and the normal enrichment of shaker-type K+ channels in these regions was severely disrupted, in the central and peripheral nervous systems. In contrast, the localization of protein 4.1B, an axoplasmic partner of Caspr2, was only moderately altered. TAG-1, which is expressed in both neurons and glia, was able to associate in cis with Caspr2 and in trans with itself. Thus, a tripartite intercellular protein complex, comprised of these two proteins, appears critical for axo-glial contacts at juxtaparanodes. This complex is analogous to that described previously at paranodes, suggesting that similar molecules are crucial for different types of axo-glial interactions.
Figures
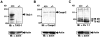
Similar articles
-
Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1.J Cell Biol. 2003 Sep 15;162(6):1149-60. doi: 10.1083/jcb.200305018. Epub 2003 Sep 8. J Cell Biol. 2003. PMID: 12963709 Free PMC article.
-
Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon.J Neurosci. 2001 Oct 1;21(19):7568-75. doi: 10.1523/JNEUROSCI.21-19-07568.2001. J Neurosci. 2001. PMID: 11567047 Free PMC article.
-
Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres.Eur J Neurosci. 2003 Jan;17(2):411-6. doi: 10.1046/j.1460-9568.2003.02441.x. Eur J Neurosci. 2003. PMID: 12542678
-
Glial regulation of the axonal membrane at nodes of Ranvier.Curr Opin Neurobiol. 2006 Oct;16(5):508-14. doi: 10.1016/j.conb.2006.08.003. Epub 2006 Sep 1. Curr Opin Neurobiol. 2006. PMID: 16945520 Review.
-
GPI-anchored proteins at the node of Ranvier.FEBS Lett. 2010 May 3;584(9):1787-92. doi: 10.1016/j.febslet.2009.08.025. Epub 2009 Aug 22. FEBS Lett. 2010. PMID: 19703450 Review.
Cited by
-
Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development.Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18120-5. doi: 10.1073/pnas.1216398109. Epub 2012 Oct 16. Proc Natl Acad Sci U S A. 2012. PMID: 23074245 Free PMC article.
-
Myelin damage and repair in pathologic CNS: challenges and prospects.Front Mol Neurosci. 2015 Jul 27;8:35. doi: 10.3389/fnmol.2015.00035. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26283909 Free PMC article. Review.
-
Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System.Physiol Rev. 2017 Apr;97(2):839-887. doi: 10.1152/physrev.00010.2016. Physiol Rev. 2017. PMID: 28298428 Free PMC article. Review.
-
Characterisation of CASPR2 deficiency disorder--a syndrome involving autism, epilepsy and language impairment.BMC Med Genet. 2016 Feb 3;17:8. doi: 10.1186/s12881-016-0272-8. BMC Med Genet. 2016. PMID: 26843181 Free PMC article.
-
The bone transcription factor Osterix controls extracellular matrix- and node of Ranvier-related gene expression in oligodendrocytes.Neuron. 2024 Jan 17;112(2):247-263.e6. doi: 10.1016/j.neuron.2023.10.008. Epub 2023 Nov 3. Neuron. 2024. PMID: 37924811 Free PMC article.
References
-
- Baumgartner, S., J.T. Littleton, K. Broadie, M.A. Bhat, R. Harbecke, J.A. Lengyel, R. Chiquet-Ehrisman, A. Prokop, and H.J. Bellen. 1996. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 87:1059–1068. - PubMed
-
- Bhat, M.A., J.C. Rios, Y. Lu, G.P. Garci-Fresco, W. Ching, M. St Martin, J. Li, and S. Einheber. 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 30:369–383. - PubMed
-
- Boyle, M.E., E.O. Berglund, K.K. Murai, L. Weber, E. Peles, and B. Ranscht. 2001. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 30:385–397. - PubMed
-
- Buttiglione, M., J.M. Revest, O. Pavlou, D. Karagogeos, A. Furley, G. Rougon, and C. Faivre-Sarrailh. 1998. A functional interaction between the neuronal adhesion molecules TAG-1 and F3 modulates neurite outgrowth and fasciculation of cerebellar granule cells. J. Neurosci. 18:6853–6870. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

